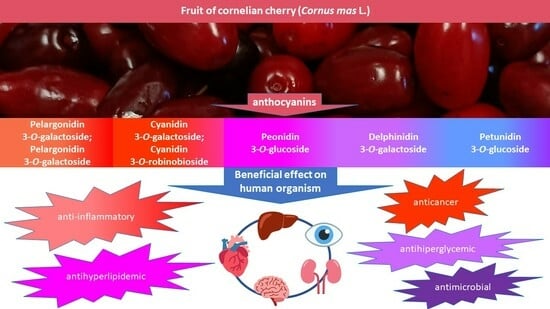
Health-Promoting Properties of Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits
Abstract
:1. Introduction
2. Methods
- population/patient/problem (P);
- intervention (I);
- comparison (C);
- outcome (O).
3. Bioavailability and Bioefficacy of Anthocyanins Derived from Cornelian Cherry
4. Health-Promotion Effects of Anthocyanins Derived from Cornelian Cherry
4.1. Anti-Inflammatory Properties
4.2. Antioxidant Properties
4.3. Protective Effects on Human Body Organs
4.3.1. The Cardioprotective Effects
4.3.2. The Liver-Protective Effects
4.3.3. The Renal Protective Effects
4.3.4. The Brain-Protective Effects
4.3.5. Vision Protective Effects
4.4. Anticarcinogenic Activity
4.5. Antihyperglycemic Effects
4.6. Hypolipidemic Effects
4.7. Antimicrobial Effects
5. Safety of Anthocyanins
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.; Liu, J.; Li, L.; Ren, J.; Lu, J.; Luo, F. Advances in Embedding Techniques of Anthocyanins: Improving Stability, Bioactivity and Bioavailability. Food Chem. X 2023, 20, 100983. [Google Scholar] [CrossRef]
- Kalt, W. Anthocyanins and Their C6-C3-C6 Metabolites in Humans and Animals. Molecules 2019, 24, 4024. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins Are Bioavailable in Humans Following an Acute Dose of Cranberry Juice. J. Nutr. 2010, 140, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- De Biaggi, M.; Donno, D.; Mellano, M.G.; Riondato, I.; Rakatoniaina, E.N.; Beccaro, G.L. Cornus mas (L.) Fruit as a Potential Source of Natural Health-Promoting Compounds: Physico-Chemical Characterisation of Bioactive Components. Plant Foods Hum. Nutr. 2018, 73, 89–94. [Google Scholar] [CrossRef]
- Hosseinpour-Jaghdani, F.; Shomali, T.; Gholipour-Shahraki, S.; Rahimi-Madiseh, M.; Rafieian-Kopaei, M. Cornus mas: A Review on Traditional Uses and Pharmacological Properties. J. Complement. Integr. Med. 2017, 14, 20160137. [Google Scholar] [CrossRef] [PubMed]
- Jing, N.; Song, J.; Liu, Z.; Wang, L.; Jiang, G. Glycosylation of Anthocyanins Enhances the Apoptosis of Colon Cancer Cells by Handicapping Energy Metabolism. BMC Complement. Med. Ther. 2020, 20, 312. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Bouzidi, C.; Marie, A.; Acquaviva, R.; Cappello, A.R.; Deguin, B. Iridoid- and Flavonoid-Enriched Fractions of Cornus sanguinea and Cornus mas Exert Antioxidant and Anti-Inflammatory Effects and Inhibit Key Enzymes in the Treatment of Metabolic Disorders. Food Funct. 2023, 14, 8838–8853. [Google Scholar] [CrossRef]
- Shih, P.-H.; Yeh, C.-T.; Yen, G.-C. Anthocyanins Induce the Activation of Phase II Enzymes through the Antioxidant Response Element Pathway against Oxidative Stress-Induced Apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef]
- Calfío, C.; Donoso, F.; Huidobro-Toro, J.P. Anthocyanins Activate Membrane Estrogen Receptors with Nanomolar Potencies to Elicit a Nongenomic Vascular Response Via NO Production. JAHA 2021, 10, e020498. [Google Scholar] [CrossRef]
- Lila, M.A. Anthocyanins and Human Health: An In Vitro Investigative Approach. J. Biomed. Biotechnol. 2004, 2004, 306–313. [Google Scholar] [CrossRef]
- Wallace, T.C. Anthocyanins in Cardiovascular Disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef]
- Lachowicz, S.; Bieniek, A.; Gil, Z.; Bielska, N.; Markuszewski, B. Phytochemical Parameters and Antioxidant Activity of New Cherry Silverberry Biotypes (Elaeagnus multiflora Thunb.). Eur. Food Res. Technol. 2019, 245, 1997–2005. [Google Scholar] [CrossRef]
- Szpadzik, E.; Krupa, T.; Molska-Kawulok, K.; Przybyłko, S. Fruit Quality and Contents of Some Bioactive Compounds in Selected Czech Sweet Cherry (Prunus avium L.) Cultivars under Conditions of Central Poland. Agriculture 2022, 12, 1859. [Google Scholar] [CrossRef]
- Unal, N.; Okatan, V.; Bilgin, J.; Kahramanoğlu, I.; Hajizadeh, H.S. Impacts of Different Planting Times on Fruit Quality and Some Bioactive Contents of Different Strawberry Cultivars. Folia Hortic. 2023, 35, 221–231. [Google Scholar] [CrossRef]
- Krupa, T.; Tomala, K. Effect of Oxygen and Carbon Dioxide Concentration on the Quality of Minikiwi Fruits after Storage. Agronomy 2021, 11, 2251. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Scariot, V. Hot and Cold Drying of Edible Flowers Affect Metabolite Patterns of Extracts and Decoctions. Folia Hortic. 2023, 35, 193–207. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Cuyamendous, C.; Bonesi, M.; Sicari, V.; Trabalzini, L.; Mitaine-Offer, A.-C.; Xiao, J.; Tundis, R. An Overview of Traditional Uses, Phytochemical Compositions and Biological Activities of Edible Fruits of European and Asian Cornus Species. Foods 2022, 11, 1240. [Google Scholar] [CrossRef] [PubMed]
- Jaćimović, V.; Božović, D.; Ercisli, S.; Bosančić, B.; Necas, T. Sustainable Cornelian Cherry Production in Montenegro: Importance of Local Genetic Resources. Sustainability 2020, 12, 8651. [Google Scholar] [CrossRef]
- Szot, I.; Łysiak, G.P.; Sosnowska, B. The Beneficial Effects of Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits and Their Possible Uses: A Review. Agriculture 2023, 14, 52. [Google Scholar] [CrossRef]
- Szot, I.; Łysiak, G.P. Effect of the Climatic Conditions in Central Europe on the Growth and Yield of Cornelian Cherry Cultivars. Agriculture 2022, 12, 1295. [Google Scholar] [CrossRef]
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Dębski, H. Anthocyanins of Fruits and Vegetables—Their Occurrence, Analysis and Role in Human Nutrition. J. Fruit. Ornam. Plant Res. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- Klymenko, S. Phenological Stages of Development of Cornus L. S. Str. Species (Cornaceae) According to BBCH Scale. Agrobiodivers Improv. Nutr. Health Life Qual. 2021, 5, 185–196. [Google Scholar] [CrossRef]
- Popović, B.M.; Štajner, D.; Slavko, K.; Sandra, B. Antioxidant Capacity of Cornelian Cherry (Cornus mas L.)—Comparison between Permanganate Reducing Antioxidant Capacity and Other Antioxidant Methods. Food Chem. 2012, 134, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Łysiak, G. Ornamental Flowers Grown in Human Surroundings as a Source of Anthocyanins with High Anti-Inflammatory Properties. Foods 2022, 11, 948. [Google Scholar] [CrossRef]
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic Effects of Extracts of Red and Yellow Fruits of Cornelian Cherries (Cornus mas L.) on Rats with Streptozotocin-Induced Diabetes Mellitus. Food Funct. 2019, 10, 6459–6472. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Silva, S.; Santos, A.L.D.; Chalfun-Júnior, A.; Zhao, J.; Peres, L.E.P.; Benedito, V.A. Understanding the Genetic Regulation of Anthocyanin Biosynthesis in Plants—Tools for Breeding Purple Varieties of Fruits and Vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Borroto Fernández, E.G.; Mokhber, A.; Zeiser, M.; Laimer, M. Phenotypic Characterization of a Wild-Type Population of Cornelian Cherries (Cornus mas L.) from Austria. Erwerbs-Obstbau 2022, 64, 673–683. [Google Scholar] [CrossRef]
- Martinović, A.; Cavoski, I. The Exploitation of Cornelian Cherry (Cornus mas L.) Cultivars and Genotypes from Montenegro as a Source of Natural Bioactive Compounds. Food Chem. 2020, 318, 126549. [Google Scholar] [CrossRef]
- Milenkovic-Andjelkovic, A.; Radovanovic, B.; Andjelkovic, M.; Radovanovic, A.; Nikolic, V.; Randjelovic, V. The Anthocyanin Content and Bioactivity of Cornelian Cherry (Cornus mas) and Wild Blackberry (Rubus fruticosus): Fruit Extracts from the Vlasina Region. Adv. Tech. 2015, 4, 26–31. [Google Scholar] [CrossRef]
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional Properties of Cornelian Cherry (Cornus mas L.): A Comprehensive Review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef]
- Antolak, H.; Czyzowska, A.; Sakač, M.; Mišan, A.; Đuragić, O.; Kregiel, D. Phenolic Compounds Contained in Little-Known Wild Fruits as Antiadhesive Agents Against the Beverage-Spoiling Bacteria Asaia spp. Molecules 2017, 22, 1256. [Google Scholar] [CrossRef]
- Ochmian, I.; Oszmiański, J.; Lachowicz, S.; Krupa-Małkiewicz, M. Rootstock Effect on Physico-Chemical Properties and Content of Bioactive Compounds of Four Cultivars Cornelian Cherry Fruits. Sci. Hortic. 2019, 256, 108588. [Google Scholar] [CrossRef]
- Sengul, M.; Eser, Z.; Ercisli, S. Chemical Properties and Antioxidant Capacity of Cornelian Cherry Genotypes Grown in Coruh Valley of Turkey. ASPHC 2014, 13, 73–82. [Google Scholar]
- Begic-Akagic, A.; Drkenda, P.; Vranac, A.; Orazem, P.; Hudina, M. Influence of Growing Region and Storage Time on Phenolic Profile of Cornelian Cherry Jam and Fruit. Eur. J. Hortic. Sci. 2013, 78, 30–39. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Brás, N.; Faria, A.; Calhau, C.; De Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 Involvement in Anthocyanin Gastric Transport- Nanobased Targeted Approach. Sci. Rep. 2019, 9, 789. [Google Scholar] [CrossRef]
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The Interaction of Anthocyanins with Bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.-L.; Rémésy, C. Anthocyanins Are Efficiently Absorbed from the Small Intestine in Rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [CrossRef]
- Mallery, S.R.; Budendorf, D.E.; Larsen, M.P.; Pei, P.; Tong, M.; Holpuch, A.S.; Larsen, P.E.; Stoner, G.D.; Fields, H.W.; Chan, K.K.; et al. Effects of Human Oral Mucosal Tissue, Saliva and Oral Microflora on Intraoral Metabolism and Bioactivation of Black Raspberry Anthocyanins. Cancer Prev. Res. 2011, 4, 1209. [Google Scholar] [CrossRef]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and Absorption of Red Grape and Wine Anthocyanins through the Gastrointestinal Tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Keppler, K.; Humpf, H.-U. Metabolism of Anthocyanins and Their Phenolic Degradation Products by the Intestinal Microflora. Bioorganic Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Danciu, V.; Moldovan, B.; Filip, A. Effects of In Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants 2019, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Schutzki, R.; Chandra, A.; Nair, M.G. Characterization, Quantification, and Bioactivities of Anthocyanins in Cornus Species. J. Agric. Food Chem. 2002, 50, 2519–2523. [Google Scholar] [CrossRef]
- Yousefi, B.; Abasi, M.; Abbasi, M.M.; Jahanban-Esfahlan, R. Anti-Proliferative Properties of Cornus mass Fruit in Different Human Cancer Cells. Asian Pac. J. Cancer Prev. 2015, 16, 5727–5731. [Google Scholar] [CrossRef] [PubMed]
- Odžaković, B.; Sailović, P.; Bodroža, D.; Kojić, V.; Jakimov, D.; Kukrić, Z. Bioactive Components and Antioxidant, Antiproliferative, and Antihyperglycemic Activities of Wild Cornelian Cherry (Cornus mas L.). Maced. J. Chem. Chem. Eng. 2021, 40, 221. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin Secretion by Bioactive Anthocyanins and Anthocyanidins Present in Fruits. J. Agric. Food Chem. 2005, 53, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Świerczewska, A.; Buchholz, T.; Melzig, M.F.; Czerwińska, M.E. In Vitro α-Amylase and Pancreatic Lipase Inhibitory Activity of Cornus mas L. and Cornus alba L. Fruit Extracts. J. Food Drug Anal. 2019, 27, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Zarei, L.; Sadrkhanlou, R.; Shahrooz, R.; Malekinejad, H.; Eilkhanizadeh, B.; Ahmadi, A. Protective Effects of Vitamin E and Cornus mas Fruit Extract on Methotrexate-Induced Cytotoxicity in Sperms of Adult Mice. Vet. Res. Forum 2014, 5, 21–27. [Google Scholar]
- Eshaghi, M.; Zare, S.; Banihabib, N.; Nejati, V.; Farokhi, F.; Mikaili, P. Cardioprotective Effect of Cornus mas Fruit Extract against Carbon Tetrachloride Induced-Cardiotoxicity in Albino Rats. J. Basic Appl. Sci. Res. 2012, 2, 11106–11114. [Google Scholar]
- Moayed Alavian, S.; Banihabib, N.; Es. Haghi, M.; Panahi, F. Protective Effect of Cornus mas Fruits Extract on Serum Biomarkers in CCl4-Induced Hepatotoxicity in Male Rats. Hepat. Mon. 2014, 14, e10330. [Google Scholar] [CrossRef]
- Somi, M.H.; Banihabib, N.; Dehghan, G.; Es. Haghi, M.; Panahi, F. Hepatoprotective Effect of Cornus mas Fruits Extract Against Carbon Tetrachloride-Induced Hepatic Damage in Male Albino Rats. Thrita 2014, 3, e17625. [Google Scholar] [CrossRef]
- Saei, H.; Hatami, H.; Azarmi, M.; Dehghan, G. Hepatoprotective Effect of Cornus mas Fruit Extract on Serum Biomarkers in Methotrexate-Induces Liver Injury in Male Rats. Pharmacol. Line 2016, 1, 91–98. [Google Scholar]
- Es Hagi, M.; Dehghan, G.; Banihabib, N.; Zare, S.; Mikalili, P.; Panahi, F. Protective Effects of Cornus mas Fruit Extract on Carbon Tetrachloride Induced Nephrotoxicity in Rats. Indian. J. Nephrol. 2014, 24, 291. [Google Scholar] [CrossRef]
- Francik, R.; Kryczyk, J.; Krośniak, M.; Berköz, M.; Sanocka, I.; Francik, S. The Neuroprotective Effect of Cornus mas on Brain Tissue of Wistar Rats. Sci. World J. 2014, 2014, 847368. [Google Scholar] [CrossRef]
- Darbandi, N.; Hashemi, A.; Noori, M.; Momeni, H.R. Effect of Cornus mas Fruit Flavonoids on Memory Retention, Level of Plasma Glucose and Lipids in an Intracerebroventricular Streptozotocin-Induced Experimental Alzheimer’s Disease Model in Wistar Rats. EEB 2016, 14, 113–120. [Google Scholar] [CrossRef]
- Omelka, R.; Blahova, J.; Kovacova, V.; Babikova, M.; Mondockova, V.; Kalafova, A.; Capcarova, M.; Martiniakova, M. Cornelian Cherry Pulp Has Beneficial Impact on Dyslipidemia and Reduced Bone Quality in Zucker Diabetic Fatty Rats. Animals 2020, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.; Matuszewska, A.; Szeląg, A.; Danielewski, M.; Dziewiszek, W.; Nikodem, A.; Filipiak, J.; Jędrzejuk, D.; Bolanowski, M.; Kucharska, A.Z.; et al. Cornelian cherry (Cornus mas L.) Extract Reduces Cardiovascular Risk and Prevents Bone Loss in Ovariectomized Wistar Rats. J. Funct. Foods 2022, 90, 104974. [Google Scholar] [CrossRef]
- Asgary, S.; Rafieian-Kopaei, M.; Shamsi, F.; Najafi, S.; Sahebkar, A. Biochemical and Histopathological Study of the Anti-Hyperglycemic and Anti-Hyperlipidemic Effects of Cornelian cherry (Cornus mas L.) in Alloxan-Induced Diabetic Rats. J. Complement. Integr. Med. 2014, 11, 63–69. [Google Scholar] [CrossRef]
- Capcarova, M.; Kalafova, A.; Schwarzova, M.; Schneidgenova, M.; Svik, K.; Prnova, M.S.; Slovak, L.; Kovacik, A.; Lory, V.; Zorad, S.; et al. Cornelian Cherry Fruit Improves Glycaemia and Manifestations of Diabetes in Obese Zucker Diabetic Fatty Rats. Res. Vet. Sci. 2019, 126, 118–123. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.-H.; Nair, M.G. Amelioration of Obesity and Glucose Intolerance in High-Fat-Fed C57BL/6 Mice by Anthocyanins and Ursolic Acid in Cornelian cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Ląd, A.; Woźniak, A.; Dzimira, S.; Piórecki, N.; Trocha, M. The Protective Effect of the Cornus mas Fruits (Cornelian cherry) on Hypertriglyceridemia and Atherosclerosis through PPARα Activation in Hypercholesterolemic Rabbits. Phytomedicine 2014, 21, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.; Dzimira, S.; Magdalan, J.; Szumny, D.; Matuszewska, A.; Nowak, B.; Piórecki, N.; Szeląg, A.; Trocha, M. Loganic Acid and Anthocyanins from Cornelian cherry (Cornus mas L.) Fruits Modulate Diet-Induced Atherosclerosis and Redox Status in Rabbits. Adv. Clin. Exp. Med. 2018, 27, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid–Loganic Acid versus Anthocyanins from the Cornus mas Fruits (Cornelian cherry): Common and Different Effects on Diet-Induced Atherosclerosis, PPARs Expression and Inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Danielewski, M.; Kucharska, A.Z.; Matuszewska, A.; Rapak, A.; Gomułkiewicz, A.; Dzimira, S.; Dzięgiel, P.; Nowak, B.; Trocha, M.; Magdalan, J.; et al. Cornelian cherry (Cornus mas L.) Iridoid and Anthocyanin Extract Enhances PPAR-α, PPAR-γ Expression and Reduces I/M Ratio in Aorta, Increases LXR-α Expression and Alters Adipokines and Triglycerides Levels in Cholesterol-Rich Diet Rabbit Model. Nutrients 2021, 13, 3621. [Google Scholar] [CrossRef]
- Asgary, S.; Rafieian-Kopaei, M.; Adelnia, A.; Kazemi, S.; Shamsi, F. Comparing the Effects of Lovastatin and Cornus mas Fruit on Fibrinogen Level in Hypercholesterolemic Rabbits. ARYA Atheroscler. 2010, 6, 1–5. [Google Scholar]
- Abdollahi, B.; Mesgari Abbasi, M.; Zakeri Milani, P.; Sadat Nourdadgar, A.; Banan Khojasteh, S.M.; Nejati, V. Hydro-Methanolic Extract of Cornus mas L. and Blood Glucose, Lipid Profile and Hematological Parameters of Male Rats. Iran. Red. Crescent Med. J. 2014, 16, e17784. [Google Scholar] [CrossRef]
- Szumny, D.; Sozański, T.; Kucharska, A.Z.; Dziewiszek, W.; Piórecki, N.; Magdalan, J.; Chlebda-Sieragowska, E.; Kupczynski, R.; Szeląg, A.; Szumny, A. Application of Cornelian Cherry Iridoid-Polyphenolic Fraction and Loganic Acid to Reduce Intraocular Pressure. Evid.-Based Complement. Altern. Med. 2015, 2015, 939402. [Google Scholar] [CrossRef]
- Sangsefidi, Z.S.; Hosseinzadeh, M.; Ranjbar, A.M.; Akhondi-Meybodi, M.; Fallahzadeh, H.; Mozaffari-Khosravi, H. The Effect of Total Anthocyanin-Base Standardized (Cornus mas L.) Fruit Extract on Liver Function, Tumor Necrosis Factor α, Malondealdehyde, and Adiponectin in Patients with Non-Alcoholic Fatty Liver: A Study Protocol for a Double-Blind Randomized Clinical Trial. Nutr. J. 2019, 18, 39. [Google Scholar] [CrossRef]
- Sangsefidi, Z.S.; Yarhosseini, F.; Hosseinzadeh, M.; Ranjbar, A.; Akhondi-Meybodi, M.; Fallahzadeh, H.; Mozaffari-Khosravi, H. The Effect of (Cornus mas L.) Fruit Extract on Liver Function among Patients with Nonalcoholic Fatty Liver: A Double-blind Randomized Clinical Trial. Phytother. Res. 2021, 35, 5259–5268. [Google Scholar] [CrossRef]
- Soltani, R.; Gorji, A.; Asgary, S.; Sarrafzadegan, N.; Siavash, M. Evaluation of the Effects of Cornus mas L. Fruit Extract on Glycemic Control and Insulin Level in Type 2 Diabetic Adult Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Evid.-Based Complement. Altern. Med. 2015, 2015, 740954. [Google Scholar] [CrossRef]
- Asgary, S.; Kelishadi, R.; Rafieian-Kopaei, M.; Najafi, S.; Najafi, M.; Sahebkar, A. Investigation of the Lipid-Modifying and Antiinflammatory Effects of Cornus mas L. Supplementation on Dyslipidemic Children and Adolescents. Pediatr. Cardiol. 2013, 34, 1729–1735. [Google Scholar] [CrossRef]
- Yarhosseini, F.; Sangouni, A.A.; Sangsefidi, Z.S.; Hosseinzadeh, M.; Akhondi-Meybodi, M.; Ranjbar, A.; Fallahzadeh, H.; Mozaffari-Khosravi, H. Effect of Cornus mas L. Fruit Extract on Blood Pressure, Anthropometric and Body Composition Indices in Patients with Non-Alcoholic Fatty Liver Disease: A Double-Blind Randomized Controlled Trial. Clin. Nutr. ESPEN 2023, 56, 18–24. [Google Scholar] [CrossRef]
- Dadkhah, N.; Shirani, M.; Etemadifar, S.; Mirtalebi, M. The Effect of Cornus mas in Preventing Recurrent Urinary Tract Infections in Women. Future Nat. Prod. 2017, 3, 67–76. [Google Scholar]
- Seeram, N. Cyclooxygenase Inhibitory and Antioxidant Cyanidin Glycosides in Cherries and Berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.-C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and Antiinflammatory Activities of Anthocyanins and Their Aglycon, Cyanidin, from Tart Cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Szaniawska, M.; Taraba, A.; Szymczyk, K. Structure, Properties and Application of Anthocyanins. Eng. Sci. Technol. 2015, 2, S63–S78. [Google Scholar] [CrossRef]
- Sarma, A.D.; Sharma, R. Anthocyanin-DNA Copigmentation Complex: Mutual Protection against Oxidative Damage. Phytochemistry 1999, 52, 1313–1318. [Google Scholar] [CrossRef]
- Szczepaniak, O.; Ligaj, M.; Stuper-Szablewska, K.; Kobus-Cisowska, J. Genoprotective Effect of Cornelian Cherry (Cornus mas L.) Phytochemicals, Electrochemical and Ab Initio Interaction Study. Biomed. Pharmacother. 2022, 152, 113216. [Google Scholar] [CrossRef]
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic Effects of Anthocyanins for Vision and Eye Health. Molecules 2019, 24, 3311. [Google Scholar] [CrossRef]
- Renis, M.; Calandra, L.; Scifo, C.; Tomasello, B.; Cardile, V.; Vanella, L.; Bei, R.; Fauci, L.L.; Galvano, F. Response of Cell Cycle/Stress-Related Protein Expression and DNA Damage upon Treatment of CaCo2 Cells with Anthocyanins. Br. J. Nutr. 2008, 100, 27–35. [Google Scholar] [CrossRef]
- Wang, L.-S.; Stoner, G.D. Anthocyanins and Their Role in Cancer Prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Łysiak, G.P.; Szot, I. The Possibility of Using Fruit-Bearing Plants of Temperate Climate in the Treatment and Prevention of Diabetes. Life 2023, 13, 1795. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Du, H.; Wang, L.; Li, S.; Zhang, L.; Zhang, L. Nitrite Scavenging and Inhibition of N -Nitrosamines Formation by Phenolic Extracts From Diospyros Lotus L. Leaves and Active Ingredients. Nat. Prod. Commun. 2020, 15, 1934578X2096118. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Asgary, S.; Adelnia, A.; Setorki, M.; Khazaei, M.; Somayek, K.; Shamsi, F. The Effects of Cornelian Cherry on Atherosclerosis and Atherogenic Factors in Hypercholesterolemic Rabbits. J. Med. Plants Res. 2011, 5, 2670–2676. [Google Scholar]
- Liu, C.; Sun, J.; Lu, Y.; Bo, Y. Effects of Anthocyanin on Serum Lipids in Dyslipidemia Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0162089. [Google Scholar] [CrossRef]
- Krzyściak, P.; Krośniak, M.; Gąstoł, M.; Ochońska, D.; Krzyściak, W. Antimicrobial Activity of Cornelian Cherry (Cornus mas L.). Postępy Fitoter. 2011, 4, 227–231. [Google Scholar]
- West, B.J.; Deng, S.; Jensen, C.J.; Palu, A.K.; Berrio, L.F. Antioxidant, Toxicity, and Iridoid Tests of Processed Cornelian Cherry Fruits: Tests of Processed Cornelian Cherry Fruits. Int. J. Food Sci. Technol. 2012, 47, 1392–1397. [Google Scholar] [CrossRef]
- European Food Safety Authority Scientific Opinion on the Re-Evaluation of Anthocyanins (E 163) as a Food Additive. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2013.3145 (accessed on 23 May 2023).
- Srinivas, N.R. Cranberry Juice Ingestion and Clinical Drug-Drug Interaction Potentials; Review of Case Studies and Perspectives. J. Pharm. Pharm. Sci. 2013, 16, 289. [Google Scholar] [CrossRef]


| Health-Promoting Properties | Literature | Cells Used in the Study (Population) | Study Treatment (Intervention) | Control Treatment (Comparison) | Main Findings (Outcome) |
|---|---|---|---|---|---|
| Anti-inflammatory activity | [43] | Ram seminal vesicles | Anthocyanins of C. mas—juice: delphinidin 3-O-‚-galactopyranoside 280 ppm, cyanidin 3-O-‚galactopyranoside 1079 ppm, and pelargonidin 3-O-‚-galactopyranoside 710 ppm | Ibuprofen, naproxen, | Ibuprofen and naproxen showed 47.5 and 54.3% of COX-I and 39.8 and 41.3% of COX-II inhibitory activities, respectively, at 10 μM concentrations. Anthocyanins 1, 2, and 3 displayed 9.2, 7.6, and 5.3% COX-I and 11.7, 12.4, and 7.8% COX-II activities, respectively. |
| Anticancer activity | [44] | Lung non-small cell cancer; breast adenocarcinoma cell; ovarian cancer cell; prostate adenocarcinoma cell | Hydro-alcoholic extract of C. mas: 0, 5, 20, 100, 250, 500, 1000 μg/mL | Negative control (cells in RPMI-1640 medium) | The mean growth inhibition was 81.8%, 81.9%, 81.6%, and 79.3% in ovarian cancer, breast adenocarcinoma, prostate adenocarcinoma, and lung non-small cell cancer, respectively. |
| [45] | Breast adenocarcinoma, cervix epithelioid carcinoma, lung adenocarcinoma | containing different amounts of anthocyanins depending on growth locations CC1 0.89, CC2 0.80, CC3 1.40, CC4 1.08 mg CyGE·g−1 FW | Negative control (Cells in DMEM medium) (PAA Laboratories GmbH, Pashing, Austria) | The antiproliferative activity of cornelian cherry fruit extracts depended on growth locations. Wild cornelian cherry (CC3) from Drinić had the highest monomeric anthocyanin content and the highest inhibition of free radicals (IC50DPPH = 262.19 mg/mL; IC50ABTS = 76.78 mg/mL; IC50OH˙ = 102.31 mg/mL) and inhibition of breast adenocarcinoma cell line growth (IC50MCF-7 = 1.37 mg/mL). | |
| Antihyperglycemic effects | [46] | Rodent pancreatic β-cells (INS-1 832/13) | Purified delphinidin-3-glucoside from C. officinalis fruits. cyanidin-3-galactoside and pelargonidin-3-galactoside from C. mas fruits: 5, 10, 50, 100 and 250 μg·mL−1. | Negative control (cells in RPMI-1640 medium) | Delphinidin 3-O-glucoside, cyanidin 3-O-galactoside, and pelargonidin 3-O-galactoside were distinguished as the most effective anthocyanins to stimulate insulin secretion. |
| Antihyperlipidemic effects | [47] | Porcine pancreas powder | Water and ethanolic extract of C. mas and C. alba; pelargonidin 3-O-galactoside: 7.5 μg·mL−1 | Negative control (Porcine pancreas powder in Tris-HCl buffer, pH 8.0) | The most active anthocyanin in the inhibition of pancreatic lipase activity was pelargonidin 3-O-galactoside. |
| Effects | Literature | Animals Subjected to Tests (Population) | Study Treatment (Intervention) | Control Treatment (Comparator) | Main Findings (Outcome) |
|---|---|---|---|---|---|
| Antioxidant activity | [48] | 8–12 weeks male NMRI mice treated with Methotrexate (MTX) | CMFE (250, 500, 1000 mg·kg−1) and Vitamin E (100 IU kg−1) | physiologic saline | Both Vit E and CMFE were able to protect from MTX-induced effects on sperm maturity and DNA damage. |
| Protective effect on the heart | [49] | Rats treated with carbon tetrachloride (CCl4) | Pre and post-treatment CMFE 300 and 700 mg·kg−1 | Control group without CMFE | CMFE significantly decreased the increased levels of serum lactate dehydrogenase, serum creatine kinase, and myocardial lipid peroxides and significantly increased the myocardial endogenous antioxidants (glutathione peroxidase, superoxide dismutase, and catalase) levels. |
| Protective effect on the liver | [50] | Wistar strain male albino rats treated with carbon tetrachloride | CMFE 200 and 500 mg·kg−1 and Silymarin 100 mg·kg−1 | irrigated with drinking water | Oral administration of CMFE provided significant liver protection by reducing elevated serum enzyme levels, total serum protein, albumin, and hepatic lipid peroxidation content. |
| [51] | Wistar strain male albino rats treated with carbon tetrachloride | CMFE 200 and 500 mg·kg−1 per 16 days and CMFE 200 and 500 mg·kg−1 administered 2, 6, 12, 24 and 48 h after CCl4 toxication | irrigated with drinking water | The activities of antioxidant enzymes (MDA, CAT, SOD, GPx) in the CCl4-treated group were lower than those in the normal control. The activity of these enzymes in the CMFE-treated groups increased significantly compared to the toxic group. | |
| [52] | Wistar strain male albino rats treated with methotrexate (MTX) | CMFE 700 mg·kg−1 per 7 days and Mice first day treated with MTX and then treated with 300, 700, and 1400 mg for 7 days | physiologic saline | Rats treated with MTX were characterized by significantly higher total bilirubin values and higher AST, ALT, and ALB values compared to rats treated with CMFE. The most beneficial effect on the normalization of the above-mentioned parameters after MTX administration was CMFE treatment at a dose of 1400 mg·kg−1. | |
| Protective effect on the kidneys | [53] | Wistar strain male albino rats treated with carbon tetrachloride- | Prophylactic groups: CMFE 300 and 700 mg·kg−1, for 16 days, respectively and on the 16th day received CCl4 and curative groups: distilled water orally for 16 days and on the 16th day they received CCl4 (1 mL·kg−1 b.w.; 80% in olive oil), followed by CMFE 300 mg·kg−1 and 700 mg·kg−1, respectively, at 2, 6, 12, 24 and 48 h after CCl4 intoxication | “Sham” control for both preventive and therapeutic studies, receiving raw water and free access to food for 16 days and control for both preventive and therapeutic studies, receiving distilled water orally for 16 days, and on day 16 received olive oil daily (1 mL kg−1 b.w.) | Different doses of fruit extract (300 and 700 mg/kg−1) significantly ameliorated the alterations induced by CCl4 in lipid peroxidation, antioxidant defenses, and biochemical and renal lesions. The level of antioxidant enzymes such as SOD, CAT, and GPx decreased in the CCl4-treated group and improved by treatment with CMFE. |
| Protective effect on the brain | [54] | 12-week Wistar strain male albino rats | Rats with fructose diet (with CMFE) | Rats with a diet enriched in fat | Addition of CMFE stimulates PON activity, both in brain tissue and in plasma, and increases the protection of the nervous system from oxidative stress by increasing the activity of CAT. Protects proteins against peroxidation, as can be shown by the level of PCG. |
| [55] | Wistar rats with streptozotocin-induced Alzheimer’s Disease | Flavonoid from CMFE at 5, 10 and 20 mg·kg−1 | saline-saline control, streptozotocine-saline control | CMFE treatment increased memory retention in a dose-dependent manner. The dose of 10 mg kg–1 decreased rat weight significantly. | |
| Protective effect on bones | [56] | Zucker diabetic fatty (ZDF) rats | diabetic obese rats receiving 500 and 1000 mg·kg−1 b.w. of CMFE | non-diabetic lean rats | A higher dose of CMFE had a beneficial impact on femoral weight, cortical bone thickness, relative volume of trabecular bone, and trabecular thickness. |
| [57] | 12-week-old female Wistar Rats with ovariectomy-induced metabolic changes | ovariectomized animals receiving 17β-oestradiol; group with CMFE (50 mg·kg−1) | “Sham” operated group and ovariectomized control group | CMFE ameliorated ovariectomy-induced decrease in femoral and tibial bone mineral density (BMD), prevented the deterioration in Young’s modulus and flexural strength and counteracted ovariectomy-induced decrease in serum calcium level. | |
| Antihyperglycemic | [58] | Adult male rats with alloxan-induced diabetes | glibenclamide-treated (0.6 mg·kg−1·day−1; 4 weeks) and CMFE-treated (2 g−1 day; 4 weeks) group | non-diabetic control and diabetic control | Diabetic rats had significantly elevated levels of serum glucose, LDL-C, TG, AST, ALP, and ALT and decreased levels of HDL-C compared to the non-diabetic group. The effects of CMFE were comparable to those of glibenclamide at the doses tested in this study. |
| [59] | Zucker diabetic fatty (ZDF) rats | CMFE in two doses (500 and 1000 mg·kg−1 b.w., 10 weeks) | non-diabetic lean controls received only distilled water | significant decrease of glucose level after oral administration of CMFE in a dose of 1000 mg/kg bw in the pre-diabetic state of animals (until the 7th week of the experiment) and significant restriction of water intake in both CMFE groups against the diabetic control. | |
| [25] | Male Wistar rats with streptozotocin-induced diabetes mellitus | CMFE extracts (20 mg kg−1 of b.w., 14 days) | control group (healthy) | CMFE lowered blood glucose and improved glucose tolerance. Significantly decreased the amount of glycated hemoglobin (by 25%) and increased erythrocyte resistance to acid hemolysis. | |
| Antihyperlipidemic effects | [60] | C57BL/6 mice fed a high-fat diet | mice were fed with a high-fat diet plus anthocyanins (1 g·kg−1) or ursolic acid 500 mg·kg−1) | mice were fed a normal diet | The anthocyanin showed a 24% decrease in weight gain and decreased lipid accumulation in the liver, including a significant decrease in liver triacylglycerol concentration. Anthocyanin and ursolic acid have extremely elevated insulin levels. |
| [58] | Adult male rats with alloxan-induced diabetes mellitus | glibenclamide-treated (0.6 mg/kg/day; 4 weeks) and CMFE (2 g·day−1; 4 weeks) group | non-diabetic control and diabetic control, | Treatment with glibenclamide or CM counterbalanced significantly increased serum glucose, LDL-C, TG, AST, ALP, and ALT levels and decreased HDL-C levels in diabetic rats. | |
| [61,62,63] | Adult male New Zealand rabbits with high cholesterol (1%) diet-induced hyperlipidemia | CMFE (100 mg·kg−1 b.w., 60 days) or simvastatin (5 mg·kg−1 b.w., 60 days) or loganic acid (20 mg·kg−1 b.w.) | control group with a standard diet and group with hyperlipidemia | CMFE led to a 44% significant decrease in serum TG levels and prevented the development of atheromatous changes in the thoracic aorta. CMFE significantly increased PPARα, had a significant protective effect on diet-induced oxidative stress in the liver, as well as restored upregulated pro-inflammatory cytokines serum levels. | |
| [64] | Adult male New Zealand rabbits with high cholesterol (1%) diet-induced hyperlipidemia | CMFE (10 or 50 mg·kg−1 b.w.) or simvastatin (5 mg·kg−1 b.w.) | control group with a standard diet for rabbits and a group with hyperlipidemia | CMFE enhancement in PPAR-α and PPAR-γ expression in the aorta, LXR-α expression in the liver, a decrease in TG, leptin, and resistin, and an increase in adiponectin levels. | |
| [65] | Adult male New Zealand rabbits with high cholesterol (1%) diet-induced hyperlipidemia | Group with a standard diet containing C. mas powder (1 g·kg−1 b.w.) diet, and with a high cholesterol (1%) containing C. mas powder (1 g·kg−1) diet, and a high cholesterol containing lovastatin (10 mg·kg−1 b.w.) diet | control group with a standard diet for rabbits and with a high cholesterol (1%) diet | C. mas powder and lovastatin significantly decreased fibrinogen levels in comparison with the high cholesterol group. Furthermore, C mas. powder could reduce the fibrinogen level more than lovastatin | |
| [66] | Healthy male Wistar rats | CMFE (50, 200 and 400 mg·kg−1 b.w., 3 weeks) | Normal control with normal diet without any injection and placebo control and intraperitoneally received a normal saline | All doses of CMFE significantly decreased HDW and PDW vs. the control group. Only high doses caused a significant elevation in MCHC, MPV, and PCT and a significant decrease in RDW vs. the control group. | |
| Protective effect on the eyes | [67] | Sexually mature, New Zealand white rabbits, aged between 6 and 12 months, were used: 7 males and 7 females | Intraconjunctival administration of one drop loganic acid or polyphenolic fraction of C. mas, corresponding to a volume of 50 µL, to the right eye | One drop of artificial tears containing 0.15% sodium hyaluronate (50 µL) was administered to the left eye as a placebo | Loganic acid (50%) and pelargonidin-3-galactoside (7%) were found as the main components of C. mas fraction. Therefore, the hypotensive effect was attributed to loganic acid. |
| Effects | Literature | Type of Patients (Population) | Dose of Cornelian Cherry Extract and Period of Its Intake (Intervention) | Control Treatment (Comparator) | Main Findings (Outcome) |
|---|---|---|---|---|---|
| Protective effect on the liver | [68] | Patients with non-alcoholic fatty liver disease (NAFLD) | CMFE (320 mg·d−1 anthocyanins; 12 weeks). | Control group received the placebo, matched with the extract in terms of appearance, taste, color, and texture (but without any anthocyanins) for 12 weeks | Results indicated that anthocyanins had some impacts on NAFLD. |
| [69] | Patients with non-alcoholic fatty liver disease (NAFLD) | CMFE (320 mg·d−1 anthocyanins; 12 weeks). | Control group received the placebo, matched with the extract in terms of appearance, taste, color, and texture (but without any anthocyanins) for 12 weeks | No significant impact of CMFE on serum ALT and AST levels, as well as hepatic steatosis among NAFLD patients. A significant reduction was observed in the levels of CK-18 among the CMFE group at the end of the study. No significant difference was found between the CMFE and placebo groups with regard to this marker. Fibrosis score increased significantly in the placebo group at the end of the study. | |
| Antihyperglycemic | [70] | Patients with type 2 diabetes mellitus | 300 mg d−1 anthocyanins; 6 weeks | Placebo capsules; 6 weeks | Significant increase in insulin levels and a decrease in HgbA1 C and TG levels was observed in the drug group compared to the placebo. |
| Antihyperlipidemic effects | [71] | Dyslipidemic children and adolescents | 50 g of CMFE twice a day after lunch and dinner, 6 weeks | Diet without CMFE, 6 weeks | After week 6 of the trial, the TC, TG, LDL-C, apo B, ICAM-1, and VCAM-1 levels in the CMFE group were significantly lower, and the HDL-C and apo A-I levels were higher than at baseline. |
| [72] | Patients with non-alcoholic fatty liver disease (NAFLD) | 20 cc/d CMFE, 12 weeks | Placebo, 12 weeks | Treatment group, compared to the control group, showed a significant reduction in DBP and SBP. No difference between groups in weight, WC, HC, WHR, BFM, BFP, and FFM. Significant reduction in the treatment group compared to the control group in BFM and BFP. | |
| Protective effect on the urinary system | [73] | Women with chronic cystitis (UTIs) | C. mas tablet 500 mg·day−1, 6 month | Placebo, 12 weeks | C. mas decreases dysuria among patients with UTIs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szot, I.; Łysiak, G.P.; Sosnowska, B.; Chojdak-Łukasiewicz, J. Health-Promoting Properties of Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits. Molecules 2024, 29, 449. https://doi.org/10.3390/molecules29020449
Szot I, Łysiak GP, Sosnowska B, Chojdak-Łukasiewicz J. Health-Promoting Properties of Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits. Molecules. 2024; 29(2):449. https://doi.org/10.3390/molecules29020449
Chicago/Turabian StyleSzot, Iwona, Grzegorz P. Łysiak, Bożena Sosnowska, and Justyna Chojdak-Łukasiewicz. 2024. "Health-Promoting Properties of Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits" Molecules 29, no. 2: 449. https://doi.org/10.3390/molecules29020449