Selective Removal of Hemicellulose by Diluted Sulfuric Acid Assisted by Aluminum Sulfate
Abstract
:1. Introduction
2. Results and Discussion
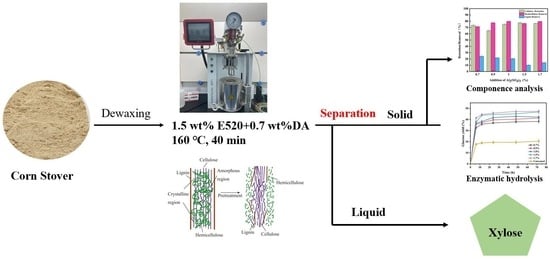
2.1. Compositional Analysis of Solid Residue of DA/E520
2.2. Compositional Analyses Liquid Fractions
2.3. Enzymatic Hydrolysis of Different Pretreatment Substrate
2.4. Characterization
2.5. Mass Balance
3. Materials and Methods
3.1. Materials
3.2. Pretreatment of Corn Stover
3.3. Analysis of Solid Residue and Liquid Fraction
3.4. Solid Residue Characterization
3.5. Enzymatic Hydrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, Z.-Y.; Li, L.; Tang, W.; Shen, J.-W.; Yang, Q.-Z.; Ma, C.; He, Y.-C. Significantly enhanced enzymatic hydrolysis of waste rice hull through a novel surfactant-based deep eutectic solvent pretreatment. Bioresour. Technol. 2023, 381, 129106. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Han, Y.; Feng, J.; Lu, X. A review about GVL production from lignocellulose: Focusing on the full components utilization. Ind. Crop. Prod. 2019, 144, 112031. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Dai, T.; Xie, J.; Zhang, H. Enhancing the co-production of sugars from sugarcane bagasse via CuCl2-catalyzed organosolv pretreatment and additives. Fuel Process. Technol. 2023, 241, 107629. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, P.; Luo, L.; Lin, H.; Tao, Y.; Ruan, L.; Wang, L.; Qing, Q. p-Toluenesulfonic acid enhanced neutral deep eutectic solvent pretreatment of soybean straw for efficient lignin removal and enzymatic hydrolysis. Bioresour. Technol. 2024, 395, 130338. [Google Scholar] [CrossRef] [PubMed]
- Moreira Neto, J.; Costa, J.M.; Bonomi, A.; Costa, A.C. A Novel Kinetic Modeling of Enzymatic Hydrolysis of Sugarcane Bagasse Pretreated by Hydrothermal and Organosolv Processes. Molecules 2023, 28, 5617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Du, X.; Qu, Y. Selective removal of lignin to enhance the process of preparing fermentable sugars and platform chemicals from lignocellulosic biomass. Bioresour. Technol. 2020, 303, 122846. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, J.S.; Sunwoo, C.; Lee, Y.Y. Pretreatment of corn stover by aqueous ammonia. Bioresour. Technol. 2003, 90, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ong, V.Z.; Yong, K.J.; Wu, T.Y. Production of aromatic monomers at one atmospheric pressure through depolymerization of lignin using combined alkaline solution and aqueous ChCl:urea. Ind. Crop. Prod. 2023, 192, 115911. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Dong, Y.; Wang, D.; Deng, J.; Shi, Z.; Yang, J.; Yang, H. Pretreatment of bamboo with choline chloride-lactic acid integrated with calcium chloride hydrates deep eutectic solvent to boost bioconversion for ethanol production. Ind. Crop. Prod. 2023, 200, 116879. [Google Scholar] [CrossRef]
- Wang, F.; Liu, B.; Cao, W.; Liu, L.; Zeng, F.; Qin, C.; Liang, C.; Huang, C.; Yao, S. Novel dual-action vanillic acid pretreatment for efficient hemicellulose separation with simultaneous inhibition of lignin condensation. Bioresour. Technol. 2023, 385, 129416. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic Extract of Propolis (EEP) Enhances the Apoptosis- Inducing Potential of TRAIL in Cancer Cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef] [PubMed]
- Parsin, S.; Kaltschmitt, M. Processing of hemicellulose in wheat straw by steaming and ultrafiltration–A novel approach. Bioresour. Technol. 2024, 393, 130071. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Singhania, R.R.; Patel, A.K.; Chen, C.-W.; Piechota, G.; Dong, C.-D. Sustainable production of cellulose and hemicellulose-derived oligosaccharides from pineapple leaves: Impact of hydrothermal pretreatment and controlled enzymatic hydrolysis. Bioresour. Technol. 2024, 398, 130526. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Tang, W.; Li, L.; Huang, M.; Ma, C.; He, Y.-C. Enhancing enzymatic hydrolysis of waste sunflower straw by clean hydrothermal pretreatment. Bioresour. Technol. 2023, 383, 129236. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, B.; Li, X.; Wang, Z.; Mu, L.; Qin, C.; Liang, C.; Huang, C.; Yao, S. Mannitol assisted oxalic acid pretreatment of poplar for the deconstruction and separation of hemicellulose. Ind. Crop. Prod. 2023, 200, 116811. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Liang, J.; Wang, F.; Bao, Y.; Qin, C.; Liang, C.; Huang, C.; Yao, S. Rapid and mild fractionation of hemicellulose through recyclable mandelic acid pretreatment. Bioresour. Technol. 2023, 382, 129154. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Wang, Z.; Qian, J.; He, Z.; Yi, S. Effects of aluminum sulfate soaking pretreatment on dimensional stability and thermostability of heat-treated wood. Eur. J. Wood Wood Prod. 2020, 79, 189–198. [Google Scholar] [CrossRef]
- Luo, Y.; Li, D.; Li, R.; Li, Z.; Hu, C.; Liu, X. Roles of water and aluminum sulfate for selective dissolution and utilization of hemicellulose to develop sustainable corn stover-based biorefinery. Renew. Sustain. Energy Rev. 2020, 122, 109724. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Huang, C.; Ling, Z.; Lai, C.; Yong, Q. Natural surfactant-aided dilute sulfuric acid pretreatment of waste wheat straw to enhance enzymatic hydrolysis efficiency. Bioresour. Technol. 2021, 324, 124651. [Google Scholar] [CrossRef] [PubMed]
- Lorenci Woiciechowski, A.; Dalmas Neto, C.J.; Porto de Souza Vandenberghe, L.; de Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance–Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhu, J.; Hou, Y.; Qin, C.; Chen, W.; Nong, Y.; Liao, Z.; Liang, C.; Bian, H.; Yao, S. Effect of temperature on simultaneous separation and extraction of hemicellulose using p-toluenesulfonic acid treatment at atmospheric pressure. Bioresour. Technol. 2022, 348, 126793. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, C.; Tang, W.; Huang, M.; Ma, C.; He, Y.-C. Enhancing enzymatic saccharification of sunflower straw through optimal tartaric acid hydrothermal pretreatment. Bioresour. Technol. 2023, 385, 129279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, B.; Yan, W.; Liu, J.; Dong, W.; Zhou, J.; Zhang, W.; Xin, F.; Jiang, M. Inhibitors tolerance analysis of Clostridium sp. strain LJ4 and its application for butanol production from corncob hydrolysate through electrochemical detoxification. Biochem. Eng. J. 2020, 167, 107891. [Google Scholar] [CrossRef]
- Gao, K.; Wang, H.; Chen, Y.; Zhang, J. Delignification of switchgrass for xylo-oligosaccharides production using sorbic acid hydrolysis. Bioresour. Technol. 2023, 385, 129390. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, B.; Liu, L.; Luo, Y.; Zeng, F.; Qin, C.; Liang, C.; Huang, C.; Yao, S. Pretreatment of poplar with eco-friendly levulinic acid to achieve efficient utilization of biomass. Bioresour. Technol. 2023, 376, 128855. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Ying, W.; Wen, P.; Lian, Z.; Zhang, J. Effect of peracetic acid generation in hydrogen peroxide-acetic acid pretreatment on production of xylooligosaccharides from poplar by organic acid hydrolysis. Bioresour. Technol. 2023, 376, 128848. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Feng, B.; Ying, W.; Lian, Z.; Zhang, J. Novel approach for corn straw biorefineries: Production of xylooligosaccharides, lignin and ethanol by nicotinic acid hydrolysis and pentanol pretreatment. Bioresour. Technol. 2024, 395, 130352. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, X.; Huang, C.; Zhan, Y.; Huang, C.; Chen, T.; Meng, X.; Yoo, C.G.; Fang, G.; Ragauskas, A.J. Novel biphasic DES/GVL solvent for effective biomass fractionation and valorization. Green Chem. 2023, 25, 6270–6281. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, R.; Song, Y.; Miu, X.; Zhang, Q.; Qu, J.; Sun, Y. Enhanced enzymatic saccharification and ethanol production of corn stover via pretreatment with urea and steam explosion. Bioresour. Technol. 2023, 376, 128856. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Li, W.; Jing, H.; Wang, M.; Li, P.; Sun, Y. Investigating the Effects of Aerobic Hydrolysis on Scum Layer Formation during the Anaerobic Digestion of Corn Stalk Particles. Sustainability 2022, 14, 6497. [Google Scholar] [CrossRef]
- Ma, Q.; Zhou, W.; Du, X.; Huang, H.; Gong, Z. Combined dilute sulfuric acid and Tween 80 pretreatment of corn stover significantly improves the enzyme digestibility: Synergistic removal of hemicellulose and lignin. Bioresour. Technol. 2023, 382, 129218. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Chen, H.; Li, Y.; Li, J.; Chen, G.; Wang, G. Pretreatment of corn stover via sodium hydroxide–urea solutions to improve the glucose yield. Bioresour. Technol. 2020, 307, 123191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, B.; Lei, F.; Li, P.; Jiang, J. Coproduction xylo-oligosaccharides with low degree of polymerization and glucose from sugarcane bagasse by non-isothermal subcritical carbon dioxide assisted seawater autohydrolysis. Bioresour. Technol. 2022, 349, 126866. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ling, Z.; Wang, C.; Zhang, X.; Xu, F. Integration of facile deep eutectic solvents pretreatment for enhanced enzymatic hydrolysis and lignin valorization from industrial xylose residue. Bioresour. Technol. 2018, 265, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, C.; Dai, T.; Liu, L.; Zhang, H.; Xie, J. Understanding the influences of poplar recalcitrance during combinatorial pretreatment on ethanol production. Fuel Process. Technol. 2023, 242, 107636. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, H.; Chen, Q.; Huang, H.; Cheng, H.; Huang, L.; Chen, L.; Ni, Y.; Cao, S. Comparison of single-stage and two-stage hydrothermal pretreatments for improving hemicellulose separation from bamboo chips. Wood Sci. Technol. 2020, 54, 547–557. [Google Scholar] [CrossRef]
- Bian, J.; Peng, F.; Peng, X.-P.; Xu, F.; Sun, R.-C.; Kennedy, J.F. Isolation of hemicelluloses from sugarcane bagasse at different temperatures: Structure and properties. Carbohydr. Polym. 2012, 88, 638–645. [Google Scholar] [CrossRef]
- Xu, C.-M.; Gao, Y.-F.; He, S.-S.; Luo, K.; Yan, Q.; Cheng, X.-Y. Improved sugar recovery from enzymatic hydrolysis of Miscanthus sinensis by surfactant-mediated alkaline pretreatment. Biomass Convers. Biorefinery 2021, 13, 4673–4680. [Google Scholar] [CrossRef]
- Zhu, J.; Jiao, N.; Li, H.; Xu, G.; Zhang, H.; Xu, Y. p-Toluenesulfonic acid combined with hydrogen peroxide-assisted pretreatment improves the production of fermentable sugars from walnut (Juglans regia L.) shells. Bioresour. Technol. 2022, 355, 127300. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, W.; Han, X.; Wang, Y.; Rao, S.; Zhang, Q.; Zhou, J.; Li, J.; Liu, S.; Du, G. Recent progress in key lignocellulosic enzymes: Enzyme discovery, molecular modifications, production, and enzymatic biomass saccharification. Bioresour. Technol. 2022, 363, 127986. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zhu, B.; Hu, Y.; Liu, Q.; Bian, J.; Li, M.; Ren, J.; Peng, F. Selectively fractionate hemicelluloses with high molecular weight from poplar thermomechanical pulp by tetramethylammonium hydroxide. Int. J. Biol. Macromol. 2024, 254, 127499. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wan, Q.; Lu, Y.; Xia, L.; Xu, J.; Gou, J. Benzoic acid catalyzed production of xylose and xylooligosaccharides from poplar. Ind. Crops Prod. 2024, 213, 118460. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, L.; Zhan, P.; Tong, Z.; Qing, Y.; He, J.; Wu, Z.; Wang, H.; Shao, L.; Liu, N. Combination of microwave with acid deep eutectic solvent pretreatment for reed (Phragmites australis) fractionation. Renew. Energy 2024, 225, 120286. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, B.; Huang, H.; Ma, C.-Y.; Ma, Y.; Shen, X.; Cao, X.; Sun, Z.; Zhang, L.; Yuan, T.-Q. Pressure-assisted hydrothermal pretreatment for biorefinery to enhance pulp production. Chem. Eng. J. 2024, 487, 150758. [Google Scholar] [CrossRef]




| Addition of E520 | T °C | t min | PY (%) | Cellulose (%) | Xylan (%) | Lignin (%) |
|---|---|---|---|---|---|---|
| Untreated | - | 36.95 ± 0.15 | 23.61 ± 0.06 | 18.04 ± 0.39 | ||
| DA | 140 | 40 | 55.97 | 59.62 ± 1.61 | 3.67 ± 0.87 | 27.02 ± 0.22 |
| 0.7% | 140 | 40 | 70.10 | 24.64 ± 0.61 | 8.57 ± 0.21 | 19.14 ± 0.21 |
| 0.9% | 70.58 | 38.81 ± 0.84 | 9.61 ± 0.16 | 19.47 ± 0.17 | ||
| 1.0% | 67.62 | 34.12 ± 0.53 | 7.51 ± 0.10 | 19.97 ± 0.31 | ||
| 1.5% | 68.90 | 40.23 ± 0.95 | 6.87 ± 0.05 | 20.75 ± 0.56 | ||
| 1.7% | 66.92 | 41.55 ± 1.23 | 8.04 ± 0.01 | 23.47 ± 0.18 | ||
| 1.5% | 150 | 61.66 | 48.30 ± 1.05 | 1.73 ± 0.17 | 28.06 ± 0.05 | |
| 160 | 61.07 | 48.85 ± 0.15 | 0.64 ± 0.15 | 27.42 ± 0.25 | ||
| 170 | 59.61 | 48.77 ± 0.78 | 0.31 ± 0.02 | 29.04 ± 0.02 | ||
| 180 | 56.25 | 45.18 ± 0.58 | 0.28 ± 0.12 | 30.14 ± 0.16 | ||
| 1.5% | 160 | 20 | 61.32 | 47.52 ± 0.07 | 1.69 ± 0.08 | 25.14 ± 0.16 |
| 30 | 61.46 | 48.31 ± 0.81 | 1.01 ± 0.10 | 25.33 ± 0.80 | ||
| 40 | 61.07 | 48.85 ± 1.22 | 0.64 ± 0.17 | 27.42 ± 0.19 | ||
| 50 | 61.47 | 46.09 ± 0.53 | 0.59 ± 0.02 | 26.96 ± 0.77 | ||
| 60 | 62.89 | 43.96 ± 1.38 | 0.50 ± 0.13 | 26.64 ± 0.29 |
| Pretreatment Condition | Sugar Content (g/100 g) | Inhibitor Content (g/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DA+E520 | T (°C) | t (min) | Total Glucose | Xylose | Xylan | Total Xylose | HMF | Furfural | Formic Acid | Acetic Acid |
| 0.7% + 0.7% E520 | 140 | 40 | 3.51 | 13.60 | 4.93 | 18.53 | 0.02 | 0.11 | 0.19 | 0.09 |
| 0.7% + 0.9% E520 | 3.67 | 14.29 | 5.41 | 19.70 | 0.02 | 0.11 | 0.19 | 0.11 | ||
| 0.7% + 1.0% E520 | 3.54 | 16.45 | 2.57 | 19.02 | 0.03 | 0.18 | 0.16 | 0.14 | ||
| 0.7% + 1.5% E520 | 3.39 | 17.77 | 2.35 | 20.12 | 0.03 | 0.24 | 0.22 | 0.17 | ||
| 0.7% + 1.7% E520 | 3.47 | 16.94 | 3.16 | 20.10 | 0.03 | 0.27 | 0.26 | 0.17 | ||
| 0.7% + 1.5% E520 | 140 | 40 | 3.39 | 17.77 | 2.35 | 20.12 | 0.03 | 0.24 | 0.22 | 0.17 |
| 150 | 3.16 | 17.27 | 2.34 | 19.61 | 0.15 | 1.26 | 1.07 | 0.47 | ||
| 160 | 3.34 | 19.44 | 1.68 | 21.12 | 0.38 | 1.87 | 3.50 | 1.11 | ||
| 170 | 3.41 | 20.02 | 1.21 | 21.23 | 0.71 | 1.94 | 8.45 | 1.74 | ||
| 180 | 3.45 | 19.99 | 1.28 | 21.27 | 0.99 | 1.76 | 12.91 | 1.71 | ||
| 0.7% + 1.5% E520 | 160 | 20 | 3.14 | 15.98 | 4.37 | 20.35 | 0.24 | 1.45 | 1.66 | 0.75 |
| 30 | 3.11 | 14.60 | 5.51 | 20.11 | 0.33 | 1.69 | 2.46 | 0.95 | ||
| 50 | 3.23 | 17.05 | 4.14 | 21.19 | 0.53 | 1.96 | 4.54 | 1.22 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Nie, J.; Zeng, L.; Zhu, F.; Gao, Z.; Zhang, A.; Xie, J.; Chen, Y. Selective Removal of Hemicellulose by Diluted Sulfuric Acid Assisted by Aluminum Sulfate. Molecules 2024, 29, 2027. https://doi.org/10.3390/molecules29092027
Jiang H, Nie J, Zeng L, Zhu F, Gao Z, Zhang A, Xie J, Chen Y. Selective Removal of Hemicellulose by Diluted Sulfuric Acid Assisted by Aluminum Sulfate. Molecules. 2024; 29(9):2027. https://doi.org/10.3390/molecules29092027
Chicago/Turabian StyleJiang, Huabin, Jiaqi Nie, Lei Zeng, Fei Zhu, Zhongwang Gao, Aiping Zhang, Jun Xie, and Yong Chen. 2024. "Selective Removal of Hemicellulose by Diluted Sulfuric Acid Assisted by Aluminum Sulfate" Molecules 29, no. 9: 2027. https://doi.org/10.3390/molecules29092027