Extraction and Isolation of Two Polysaccharides from Chloranthus japonicus Sieb. and Evaluation of Their Anti-Gastric Cancer Activities
Abstract
:1. Introduction
2. Results and Discussion
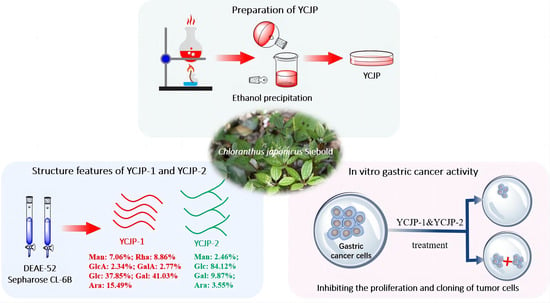
2.1. Preparation of YCJP
2.2. Isolation of YCJP–1 and YCJP–2
2.3. Structural Features of YCJP–1 and YCJP–2
2.3.1. Relative Molecular Weight Distribution
2.3.2. UV and FT-IR Analysis
2.3.3. Monosaccharide Composition Analysis
2.3.4. SEM Analysis
2.4. Anti-Gastric Cancer Activity Evaluation of YCJP, YCJP–1, and YCJP–2
2.4.1. Effects of YCJP, YCJP–1, and YCJP–2 on the Cell Viability of AGS and MGC-803 Cells
2.4.2. Effects of YCJP, YCJP–1, and YCJP–2 on the Colony and Proliferation of AGS and MGC-803 Cells
2.5. Discussion
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Crude C. japonicus Polysaccharide (YCJP)
3.3. Isolation and Purification of YCJP
3.4. Structural Features of YCJP–1 and YCJP–2
3.4.1. Relative Molecular Weight Distribution Determination
3.4.2. UV and FT-IR Spectra Analysis
3.4.3. Monosaccharide Composition Analysis
3.4.4. Scanning Electron Microscopy (SEM) Analysis
3.5. Anti-Gastric Cancer Activity Evaluation of YCJP, YCJP–1, and YCJP–2
3.5.1. Cell Culture
3.5.2. CCK-8 Assay
3.5.3. Colony-Forming Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heo, J.-E.; Jin, J.L.; Lee, Y.-Y.; YunChoi, H.-S. Chemical constituents of the aerial parts of Chloranthus japonicus Sieb. Nat. Prod. Sci. 2005, 11, 41–44. [Google Scholar]
- Liu, Y.-Y.; Li, Y.-Z.; Huang, S.-Q.; Zhang, H.-W.; Deng, C.; Song, X.-M.; Zhang, D.-D.; Wang, W. Genus Chloranthus: A comprehensive review of its phytochemistry, pharmacology, and uses. Arab. J. Chem. 2022, 15, 104260. [Google Scholar] [CrossRef]
- Kawabata, J.; Mizutani, J. Shizukanolides D, E and F, novel lindenanolides from Chlomnthus spp. (Chloranthaceae). Agric. Biol. Chem. 1989, 53, 203–207. [Google Scholar]
- Kuang, H.; Xia, Y.; Yang, B.; Wang, Q.; Lü, S. Sesquiterpene glucosides from Chloranthus japonicus Sieb. Chem. Biodivers. 2008, 5, 1736–1742. [Google Scholar] [CrossRef]
- Asgharian, P.; Quispe, C.; Herrera-Bravo, J.; Sabernavaei, M.; Rajkovic, J.; Durna Daştan, S.; Docea, A.O.; Sharifi-Rad, J.; Calina, D.; Koch, W. Pharmacological effects and therapeutic potential of natural compounds in neuropsychiatric disorders: An update. Front. Pharmacol. 2022, 13, 926607. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.-X.; Xia, Y.-G.; Yang, B.-Y.; Wang, Q.-H.; Lü, S.-W. Lignan constituents from Chloranthus japonicus Sieb. Arch. Pharmacal Res. 2009, 32, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, J.; Fukushi, Y.; Tahara, S.; Mizutani, J. Shizukaol A, a sesquiterpene dimer from Chloranthus japonicus. Phytochemistry 1990, 29, 2332–2334. [Google Scholar] [CrossRef]
- Yim, N.H.; Hwang, E.I.; Yun, B.S.; Park, K.D.; Moon, J.S.; Lee, S.H.; Do Sung, N.; Kim, S.U. Sesquiterpene Furan Compound CJ-01, a Novel Chitin Synthase 2 Inhibitor from Chloranthus japonicus Sieb. Biol. Pharm. Bull. 2008, 31, 1041–1044. [Google Scholar] [CrossRef]
- Jia, S.; Guo, R.; Huang, X.; Song, S. Research review on the main chemical constituents and pharmacological effects of Chloranthus japonicus Sieb. Asian J. Tradit. Med. 2023, 17, 275–284. [Google Scholar]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Khan, T.; Date, A.; Chawda, H.; Patel, K. Polysaccharides as potential anticancer agents—A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Zhang, L.; Tian, Q. Mushroom polysaccharide lentinan for treating different types of cancers: A review of 12 years clinical studies in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 297–328. [Google Scholar] [PubMed]
- Zhang, S.; Song, Z.; Shi, L.; Zhou, L.; Zhang, J.; Cui, J.; Li, Y.; Jin, D.Q.; Ohizumi, Y.; Xu, J.; et al. A dandelion polysaccharide and its selenium nanoparticles: Structure features and evaluation of anti-tumor activity in zebrafish models. Carbohydr. Polym. 2021, 270, 118365. [Google Scholar] [CrossRef]
- Bai, L.; Wang, T.; Deng, Q.; Zheng, W.; Li, X.; Yang, H.; Tong, R.; Yu, D.; Shi, J. Dual properties of pharmacological activities and preparation excipient: Bletilla striata polysaccharides. Int. J. Biol. Macromol. 2023, 254, 127643. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Sudharsan, S.; Sivasankar, P.; Sivakumar, L.; Vairamani, S.; Shanmugam, A. Isolation and chemical characteristics of rhamnose enriched polysaccharide from Grateloupia lithophila. Carbohyd. Polym. 2018, 195, 486–494. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Wang, X.; Huang, X.; Fei, Y.; Yu, Y.; Shou, D. Antioxidant property of water-soluble polysaccharides from Poria cocos Wolf using different extraction methods. Int. J. Biol. Macromol. 2016, 83, 103–110. [Google Scholar] [CrossRef]
- Petkova, N.; Sherova, G.; Denev, P. Characterization of inulin from dahlia tubers isolated by microwave and ultrasound-assisted extractions. Int. Food Res. J. 2018, 25, 1876–1884. [Google Scholar]
- Saha, N.; Balakrishnan, M.; Ulbricht, M. Sugarcane juice ultrafiltration: FT-IR and SEM analysis of polysaccharide fouling. J. Membrane Sci. 2007, 306, 287–297. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Mo, M.; Wang, F.; Sheng, Y.; Pan, H.; Chen, W.; Jiang, F.; Bi, Y.; Kong, F. Purification, structural elucidation and in vitro antitumor activity of a novel polysaccharide from sugarcane leaves. Ind. Crops Prod. 2024, 209, 117989. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Li, Z.; Liu, W.; Zhang, H.; Ohizumi, Y.; Nakajima, A.; Xu, J.; Guo, Y. Structure, anti-tumor activity, and potential anti-tumor mechanism of a fungus polysaccharide from Fomes officinalis. Carbohydr. Polym. 2022, 295, 119794. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Scoville, D.; He, X.C.; Mahe, M.M.; Box, A.; Perry, J.M.; Smith, N.R.; Lei, N.Y.; Davies, P.S.; Fuller, M.K. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 2013, 145, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, C.; Georgiev, M.I.; Bajpai, V.K.; Tundis, R.; Simal-Gandara, J.; Lu, X.; Xiao, J.; Tang, X.; Qiao, X. Advances in dietary polysaccharides as anticancer agents: Structure-activity relationship. Trends Food Sci. Technol. 2021, 111, 360–377. [Google Scholar] [CrossRef]
- Awadasseid, A.; Hou, J.; Gamallat, Y.; Xueqi, S.; Eugene, K.D.; Musa Hago, A.; Bamba, D.; Meyiah, A.; Gift, C.; Xin, Y. Purification, characterization, and antitumor activity of a novel glucan from the fruiting bodies of Coriolus versicolor. PLoS ONE 2017, 12, e0171270. [Google Scholar] [CrossRef] [PubMed]
- Bimczok, D.; Wrenger, J.; Schirrmann, T.; Rothkötter, H.-J.; Wray, V.; Rau, U. Short chain regioselectively hydrolyzed scleroglucans induce maturation of porcine dendritic cells. Appl. Microbiol. Biotechnol. 2009, 82, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Ooi, V.E.; Liu, F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Cur. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Xie, Z.; Huang, S.; Tai, Y.; Cai, Q.; Jiang, W.; Sun, J.; Yuan, Y. Immune-enhancing effects of polysaccharides extracted from Lilium lancifolium Thunb. Int. Immunopharmacol. 2017, 52, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.-Q.; Pan, L.-C.; Sun, H.-Q.; Ren, Y.-Y.; Zhu, Z.-Y. Structural characterization of a polysaccharide from Abelmoschus esculentus L. Moench (okra) and its hypoglycemic effect and mechanism on type 2 diabetes mellitus. Food Funct. 2022, 13, 11973–11985. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Wu, J.; Weng, L.; Zhang, H.; Zhang, J.; Wu, A. An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydr. Polym. 2020, 227, 115332. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Wang, X.; An, L.; Bao, J.; Zhang, J.; Cui, J.; Li, Y.; Jin, D.-Q.; Tuerhong, M. Isolation, structural elucidation, and immunoregulation properties of an arabinofuranan from the rinds of Garcinia mangostana. Carbohydr. Polym. 2020, 246, 116567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Q.; Li, C.; Xing, N.; Zhou, P.; Jiao, Y. A zinc-modified Anemarrhena asphodeloides polysaccharide complex enhances immune activity via the NF-κB and MAPK signaling pathways. Int. J. Biol. Macromol. 2023, 249, 126017. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiao, Y.; Shen, S.; Zhao, W.; Zhang, Q.; Zhang, S. Chaenomeles sinensis polysaccharide and its carboxymethylated derivative alleviate dextran sulfate sodium-induced ulcerative colitis via suppression of inflammation and oxidative stress. Biomed. Pharmacother. 2023, 169, 115941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; An, L.; Li, Z.; Wang, X.; Wang, H.; Shi, L.; Bao, J.; Lan, X.; Zhang, E.; Lall, N. Structural elucidation of an immunological arabinan from the rhizomes of Ligusticum chuanxiong, a traditional Chinese medicine. Int. J. Biol. Macromol. 2021, 170, 42–52. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Wang, T.; Li, C.; Tang, L.; Xiao, L. Response surface optimization of polysaccharides from jaboticaba (Myrciaria cauliflora [Mart.] O. Berg) fruits: Ultrasound-assisted extraction, structure properties, and antioxidant/hypoglycemic activities. Chem. Biodivers. 2024, 21, e202302070. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, W.; Jin, L.; Wang, Y.; Xu, X.; Ma, E.; Yang, D.; Zhao, Z. Extraction and Isolation of Two Polysaccharides from Chloranthus japonicus Sieb. and Evaluation of Their Anti-Gastric Cancer Activities. Molecules 2024, 29, 2043. https://doi.org/10.3390/molecules29092043
Liu J, Li W, Jin L, Wang Y, Xu X, Ma E, Yang D, Zhao Z. Extraction and Isolation of Two Polysaccharides from Chloranthus japonicus Sieb. and Evaluation of Their Anti-Gastric Cancer Activities. Molecules. 2024; 29(9):2043. https://doi.org/10.3390/molecules29092043
Chicago/Turabian StyleLiu, Ju, Wenfeng Li, Lu Jin, Yingchao Wang, Xinjun Xu, Enyao Ma, Depo Yang, and Zhimin Zhao. 2024. "Extraction and Isolation of Two Polysaccharides from Chloranthus japonicus Sieb. and Evaluation of Their Anti-Gastric Cancer Activities" Molecules 29, no. 9: 2043. https://doi.org/10.3390/molecules29092043