Cationic Materials for Gene Therapy: A Look Back to the Birth and Development of 2,2-Bis-(hydroxymethyl)Propanoic Acid-Based Dendrimer Scaffolds
Abstract
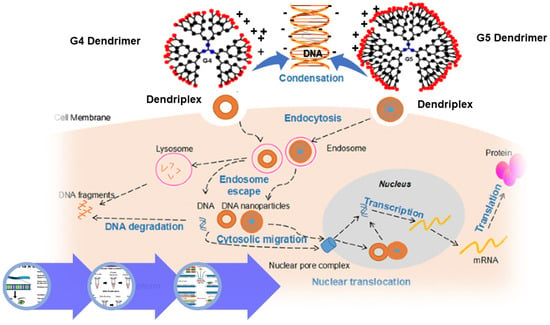
:1. Introduction
2. Gene Therapy and Non-Viral Carriers
2.1. Lipidic Carriers
2.2. Polymeric Carriers
2.2.1. Poly(L-lysine) (PLL)
2.2.2. Polyethyleneimines (PEIs)
2.2.3. Linear Poly(amidoamines) (PAA)
2.2.4. Polymethacrylates (PMA) and Polyacrylamides (PAM)
2.2.5. β-Cyclodextrins
2.2.6. Chitosans
2.2.7. Dextrans
2.3. Dendrimer Vectors
2.3.1. Polyamidoamines (PAMAM)
2.3.2. Poly(propyleneimines) (PPI)
Dendritic Polylysine (DPL)
3. Dendritic Architectures Derived from 2,2-Bis(hydroxymethyl)propanoic Acid (b-HMPA)
3.1. Strategies Used for the Synthesis of b-HMPA-Derived Dendrimer Systems
3.1.1. Conventional Approaches
Divergent Growth
Convergent Growth
3.1.2. Revisited Methods: Accelerated Approaches
Hyper Monomer
Two-Stage Convergent Growth (TSCG)
Double Exponential Growth
Procedures via Click Chemistry
3.2. Hetero Functional Dendrimer Systems
Hybrid Dendritic Systems
3.3. Dendronized Surfaces
3.4. Applications of Dendritic Materials Derived from b-HMPA
4. Our Experience
4.1. First Age Group Dendrimers
4.2. Second Age Group Dendrimers
4.3. Third Age Group Dendrimers
4.4. Fourth Age Group Dendrimers
5. Commercially Available b-HMPA-Based Dendrimers
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alfei, S.; Catena, S. Synthesis and Characterization of Versatile Amphiphilic Dendrimers Peripherally Decorated with Positively Charged Amino Acids. Polym. Int. 2018, 67, 1572–1584. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A Versatile Nanocarrier for Drug Delivery and Targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Nikzamir, M.; Hanifehpour, Y.; Akbarzadeh, A.; Panahi, Y. Applications of Dendrimers in Nanomedicine and Drug Delivery: A Review. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2246–2261. [Google Scholar] [CrossRef]
- Albertazzi, L.; Serresi, M.; Albanese, A.; Beltram, F. Dendrimer Internalization and Intracellular Trafficking in Living Cells. Mol. Pharm. 2010, 7, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Park, T.-E.; Firdous, J.; Li, H.-S.; Jimenez, Z.; Lim, M.; Choi, J.-W.; Yun, C.-H.; Cho, C.-S. Essential cues of engineered polymeric materials regulating gene transfer pathways. Prog. Mater. Sci. 2022, 128, 100961. [Google Scholar] [CrossRef]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging Non-Viral Vectors for Gene Delivery. J. Nanobiotechnol. 2023, 21, 272. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S. Synthesis and Characterization of Fourth Generation Polyester-Based Dendrimers with Cationic Amino Acids-Modified Crown as Promising Water Soluble Biomedical Devices. Polym. Adv. Technol. 2018, 29, 2735–2749. [Google Scholar] [CrossRef]
- Kesharwani, P.; Amin, M.C.I.M.; Giri, N.; Jain, A.; Gajbhiye, V. Chapter 11—Dendrimers in Targeting and Delivery of Drugs. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Mishra, V., Kesharwani, P., Mohd Amin, M.C.I., Iyer, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 363–388. ISBN 978-0-12-809717-5. [Google Scholar]
- Romani, C.; Gagni, P.; Sponchioni, M.; Volonterio, A. Selectively Fluorinated PAMAM-Arginine Conjugates as Gene Delivery Vectors. Bioconjug. Chem. 2023, 34, 1084–1095. [Google Scholar] [CrossRef]
- Morales-Sanfrutos, J.; Megia-Fernandez, A.; Hernandez-Mateo, F.; Giron-Gonzalez, M.D.; Salto-Gonzalez, R.; Santoyo-Gonzalez, F. Alkyl sulfonyl derivatized PAMAM-G2 dendrimers as nonviral gene delivery vectors with improved transfection efficiencies. Org. Biomol. Chem. 2011, 9, 851–864. [Google Scholar] [CrossRef]
- Manouchehri, S.; Zarrintaj, P.; Saeb, M.R.; Ramsey, J.D. Advanced Delivery Systems Based on Lysine or Lysine Polymers. Mol. Pharm. 2021, 18, 3652–3670. [Google Scholar] [CrossRef]
- Gorzkiewicz, M.; Konopka, M.; Janaszewska, A.; Tarasenko, I.I.; Sheveleva, N.N.; Gajek, A.; Neelov, I.M.; Klajnert-Maculewicz, B. Application of New Lysine-Based Peptide Dendrimers D3K2 and D3G2 for Gene Delivery: Specific Cytotoxicity to Cancer Cells and Transfection In Vitro. Bioorg. Chem. 2020, 95, 103504. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Castellaro, S. Synthesis and Characterization of Polyester-Based Dendrimers Containing Peripheral Arginine or Mixed Amino Acids as Potential Vectors for Gene and Drug Delivery. Macromol. Res. 2017, 25, 1172–1186. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and Biocompatible Spherical Dendrimer Nanoparticles with a Gallic Acid Shell and a Double-Acting Strong Antioxidant Activity as Potential Device to Fight Diseases from “Oxidative Stress”. Drug Deliv. Transl. Res. 2020, 10, 259–270. [Google Scholar] [CrossRef]
- Malkoch, M.; García-Gallego, S. Bis-MPA Dendrimers and Other Dendritic Polyesters. In Monographs in Supramolecular Chemistry; Malkoch, M., García-Gallego, S., Eds.; The Royal Society of Chemistry: London, UK, 2020; pp. 21–57. [Google Scholar]
- National Human Genome Research Institute. Gene Therapy. Available online: https://www.genome.gov/genetics-glossary/Gene-Therapy (accessed on 5 October 2023).
- Cring, M.R.; Sheffield, V.C. Gene Therapy and Gene Correction: Targets, Progress, and Challenges for Treating Human Diseases. Gene Ther. 2022, 29, 3–12. [Google Scholar] [CrossRef]
- Dwivedi, S.; Purohit, P.; Vasudeva, A.; Kumar, M.; Agrawal, R.; Sheikh, N.A.; Misra, R.; Kishore, S.; Misra, S. Chapter 9—Gene Therapy and Gene Editing in Healthcare. In Biotechnology in Healthcare; Barh, D., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 147–175. ISBN 978-0-323-89837-9. [Google Scholar]
- Rashid, R.A.; Ankathil, R. Gene therapy: An updated overview on the promising success stories. Malays. J. Pathol. 2020, 42, 171–185. [Google Scholar] [PubMed]
- Fus-Kujawa, A.; Prus, P.; Bajdak-Rusinek, K.; Teper, P.; Gawron, K.; Kowalczuk, A.; Sieron, A.L. An Overview of Methods and Tools for Transfection of Eukaryotic Cells In Vitro. Front. Bioeng. Biotechnol. 2021, 9, 701031. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The Current Landscape of Nucleic Acid Therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Perez Ruiz de Garibay, A. Endocytosis in Gene Therapy with Non-Viral Vectors. Wien. Med. Wochenschr. 2016, 166, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Lai, M.; Chiuppesi, F.; Ceccherini-Nelli, L.; Pistello, M. Viral vectors: A look back and ahead on gene transfer technology. New Microbiol. 2013, 36, 1–22. [Google Scholar]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral Vector Platforms within the Gene Therapy Landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-Viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Castellaro, S.; Taptue, G.B. Synthesis and NMR Characterization of Dendrimers Based on 2, 2-Bis-(Hydroxymethyl)-Propanoic Acid (Bis-HMPA) Containing Peripheral Amino Acid Residues for Gene Transfection. Org. Commun. 2017, 10, 144–177. [Google Scholar] [CrossRef]
- Thomas, M.; Klibanov, A.M. Enhancing Polyethylenimine’s Delivery of Plasmid DNA into Mammalian Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 14640–14645. [Google Scholar] [CrossRef] [PubMed]
- Aydin, O.; Kanarya, D.; Yilmaz, U.; Tunç, C.Ü. Determination of Optimum Ratio of Cationic Polymers and Small Interfering RNA with Agarose Gel Retardation Assay. Methods Mol. Biol. 2022, 2434, 117–128. [Google Scholar] [CrossRef]
- Tasset, A.; Bellamkonda, A.; Wang, W.; Pyatnitskiy, I.; Ward, D.; Peppas, N.; Wang, H. Overcoming Barriers in Non-Viral Gene Delivery for Neurological Applications. Nanoscale 2022, 14, 3698–3719. [Google Scholar] [CrossRef]
- Arévalo-Soliz, L.M.; Hardee, C.L.; Fogg, J.M.; Corman, N.R.; Noorbakhsh, C.; Zechiedrich, L. Improving therapeutic potential of non-viral minimized DNA vectors. Cell Gene Ther. Insights 2009, 6, 1489–1505. [Google Scholar] [CrossRef]
- Maffei, M.; Morelli, C.; Graham, E.; Patriarca, S.; Donzelli, L.; Doleschall, B.; de Castro Reis, F.; Nocchi, L.; Chadick, C.H.; Reymond, L.; et al. A Ligand-Based System for Receptor-Specific Delivery of Proteins. Sci. Rep. 2019, 9, 19214. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Valenti, G.E.; Domenicotti, C. Synthesis of Polystyrene-Based Cationic Nanomaterials with Pro-Oxidant Cytotoxic Activity on Etoposide-Resistant Neuroblastoma Cells. Nanomaterials 2021, 11, 977. [Google Scholar] [CrossRef]
- Bai, H.; Lester, G.M.S.; Petishnok, L.C.; Dean, D.A. Cytoplasmic Transport and Nuclear Import of Plasmid DNA. Biosci. Rep. 2017, 37, BSR20160616. [Google Scholar] [CrossRef]
- Zinchenko, A. DNA Conformational Behavior and Compaction in Biomimetic Systems: Toward Better Understanding of DNA Packaging in Cell. Adv. Colloid Interface Sci. 2016, 232, 70–79. [Google Scholar] [CrossRef]
- Paci, G.; Caria, J.; Lemke, E.A. Cargo Transport through the Nuclear Pore Complex at a Glance. J. Cell Sci. 2021, 134, jcs247874. [Google Scholar] [CrossRef]
- Tavernier, G.; Andries, O.; Demeester, J.; Sanders, N.N.; De Smedt, S.C.; Rejman, J. mRNA as Gene Therapeutic: How to Control Protein Expression. J. Control. Release 2011, 150, 238–247. [Google Scholar] [CrossRef]
- Zhao, N.; Zeng, Z.; Zu, Y. Self-Assembled Aptamer-Nanomedicine for Targeted Chemotherapy and Gene Therapy. Small 2018, 14, 1702103. [Google Scholar] [CrossRef]
- Zhang, X.-X.; McIntosh, T.J.; Grinstaff, M.W. Functional Lipids and Lipoplexes for Improved Gene Delivery. Biochimie 2012, 94, 42–58. [Google Scholar] [CrossRef]
- Petukhov, I.A.; Puchkov, P.A.; Morozova, N.G.; Zenkova, M.A.; Maslov, M.A. The Synthesis and Transfection Activity of Disulfide Polycationic Amphiphiles. Russ. J. Bioorg. Chem. 2023, 49, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Grun, M.K.; Suberi, A.; Shin, K.; Lee, T.; Gomerdinger, V.; Moscato, Z.M.; Piotrowski-Daspit, A.S.; Saltzman, W.M. PEGylation of Poly(Amine-Co-Ester) Polyplexes for Tunable Gene Delivery. Biomaterials 2021, 272, 120780. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Lipofection. Cold Spring Harb. Protoc. 2019, 2019, pdb.top096248. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Carter, M.; Shieh, J. Chapter 11—Gene Delivery Strategies. In Guide to Research Techniques in Neuroscience, 2nd ed.; Carter, M., Shieh, J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 239–252. ISBN 978-0-12-800511-8. [Google Scholar]
- Zhang, Y.; Sun, C.; Wang, C.; Jankovic, K.E.; Dong, Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef]
- Chabaud, P.; Camplo, M.; Payet, D.; Serin, G.; Moreau, L.; Barthélémy, P.; Grinstaff, M.W. Cationic Nucleoside Lipids for Gene Delivery. Bioconjug. Chem. 2006, 17, 466–472. [Google Scholar] [CrossRef]
- Mochizuki, S.; Nishina, K.; Fujii, S.; Sakurai, K. The Transfection Efficiency of Calix [4]Arene-Based Lipids: The Role of the Alkyl Chain Length. Biomater. Sci. 2015, 3, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Li, W.; Zhang, Y.; Xue, Y.; Hou, X.; Yan, J.; Cheng, J.; Deng, B.; McComb, D.W.; Lin, J.; et al. Cholesterol-Amino-Phosphate (CAP) Derived Lipid Nanoparticles for Delivery of Self-Amplifying RNA and Restoration of Spermatogenesis in Infertile Mice. Adv. Sci. 2023, 10, 2300188. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Dang, U.J.; Yuan, Y.; Psaras, A.M.; Osipitan, O.; Brooks, T.A.; Lu, F.; Di Pasqua, A.J. Optimization of DOTAP/Chol Cationic Lipid Nanoparticles for mRNA, pDNA, and Oligonucleotide Delivery. AAPS PharmSciTech 2022, 23, 135. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Nikkhah, M.; Hosseinkhani, S. Cholesterol-rich lipid-mediated nanoparticles boost of transfection efficiency, utilized for gene editing by CRISPR-Cas9. Int. J. Nanomed. 2019, 14, 4353–4366. [Google Scholar] [CrossRef] [PubMed]
- Maiti, B.; Bhattacharya, S. Liposomal Nanoparticles Based on Steroids and Isoprenoids for Nonviral Gene Delivery. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1759. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Deng, J.; Zhang, L.M. Cationic nanoparticles self-assembled from amphiphilic chitosan derivatives containing poly(amidoamine) dendrons and deoxycholic acid as a vector for co-delivery of doxorubicin and gene. Carbohydr. Polym. 2021, 258, 117706. [Google Scholar] [CrossRef]
- Kaygisiz, K.; Synatschke, C.V. Materials Promoting Viral Gene Delivery. Biomater. Sci. 2020, 8, 6113–6156. [Google Scholar] [CrossRef]
- Lee, C.; Kasala, D.; Na, Y.; Lee, M.S.; Kim, S.W.; Jeong, J.H.; Yun, C.-O. Enhanced Therapeutic Efficacy of an Adenovirus-PEI-Bile-Acid Complex in Tumors with Low Coxsackie and Adenovirus Receptor Expression. Biomaterials 2014, 35, 5505–5516. [Google Scholar] [CrossRef]
- Lostalé-Seijo, I.; Montenegro, J. Synthetic Materials at the Forefront of Gene Delivery. Nat. Rev. Chem. 2018, 2, 258–277. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, Y.; Wu, M.; Feng, S. Fluorinated Vectors for Gene Delivery. Expert Opin. Drug Deliv. 2022, 19, 1435–1448. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Sun, M.; Zhang, J.; Bi, Y. Coating Liposomes with Ring-like PEG: The Synthesis and Stealth Effect of Cholesterol–PEG–Cholesterol. Mater. Adv. 2022, 3, 2417–2424. [Google Scholar] [CrossRef]
- Laemmli, U.K. Characterization of DNA Condensates Induced by Poly(Ethylene Oxide) and Polylysine. Proc. Natl. Acad. Sci. USA 1975, 72, 4288–4292. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Y.; Wu, C.H. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J. Biol. Chem. 1987, 262, 4429–4432. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Y.; Wu, C.H. Receptor-Mediated Gene Delivery and Expression In Vivo. J. Biol. Chem. 1988, 263, 14621–14624. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, R.; Shang, Y.; Sun, L. Polylysine Complexes and Their Biomedical Applications. Eng. Regen. 2023, 4, 20–27. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Jahanpeimay Sabet, M.; Hasanzadeh, A.; Kamrani Mousavi, S.M.; Haeri Moghaddam, N.; Hooshmand, S.A.; Rabiee, N.; Liu, Y.; Hamblin, M.R.; Karimi, M. Histidine-Enhanced Gene Delivery Systems: The State of the Art. J. Gene Med. 2022, 24, e3415. [Google Scholar] [CrossRef]
- Swenson, C.S.; Lackey, H.H.; Reece, E.J.; Harris, J.M.; Heemstra, J.M.; Peterson, E.M. Evaluating the Effect of Ionic Strength on PNA:DNA Duplex Formation Kinetics. RSC Chem. Biol. 2021, 2, 1249–1256. [Google Scholar] [CrossRef]
- Oupicky, D.; Read, M.L.; Bettinger, T. Stabilization of Polycation-DNA Complexes by Surface Modification with Hydrophilic Polymers. In Nonviral Vectors for Gene Therapy: Methods and Protocols; Findeis, M.A., Ed.; Humana Press: Totowa, NJ, USA, 2001; pp. 61–78. ISBN 978-1-59259-139-8. [Google Scholar]
- McKenzie, D.L.; Smiley, E.; Kwok, K.Y.; Rice, K.G. Low Molecular Weight Disulfide Cross-Linking Peptides as Nonviral Gene Delivery Carriers. Bioconjug. Chem. 2000, 11, 901–909. [Google Scholar] [CrossRef]
- Park, S.; Healy, K.E. Nanoparticulate DNA Packaging Using Terpolymers of Poly(Lysine-g-(Lactide-b-Ethylene Glycol)). Bioconjug. Chem. 2003, 14, 311–319. [Google Scholar] [CrossRef]
- Park, S.; Healy, K.E. Compositional Regulation of Poly(Lysine-g-(Lactide-b-Ethylene Glycol))–DNA Complexation and Stability. J. Control. Release 2004, 95, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sun, Y.; Shi, Q.-S.; Liu, P.-F.; Zhu, M.-J.; Wang, C.-H.; Du, L.-F.; Duan, Y.-R. Biodegradable Nanoparticles of mPEG-PLGA-PLL Triblock Copolymers as Novel Non-Viral Vectors for Improving siRNA Delivery and Gene Silencing. Int. J. Mol. Sci. 2012, 13, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Bikram, M.; Ahn, C.-H.; Chae, S.Y.; Lee, M.; Yockman, J.W.; Kim, S.W. Biodegradable Poly(Ethylene Glycol)-Co-Poly(l-Lysine)-g-Histidine Multiblock Copolymers for Nonviral Gene Delivery. Macromolecules 2004, 37, 1903–1916. [Google Scholar] [CrossRef]
- Sanchez-Martos, M.; Martinez-Navarrete, G.; Bernabeu-Zornoza, A.; Humphreys, L.; Fernandez, E. Evaluation and Optimization of Poly-d-Lysine as a Non-Natural Cationic Polypeptide for Gene Transfer in Neuroblastoma Cells. Nanomaterials 2021, 11, 1756. [Google Scholar] [CrossRef] [PubMed]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in Gene Therapy: Current Status and Clinical Applications. J. Control. Release 2023, 362, 667–691. [Google Scholar] [CrossRef]
- Remant Bahadur, K.C.; Uludağ, H. 2—PEI and Its Derivatives for Gene Therapy. In Polymers and Nanomaterials for Gene Therapy; Narain, R., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 29–54. ISBN 978-0-08-100520-0. [Google Scholar]
- Saqafi, B.; Rahbarizadeh, F. Effect of PEI Surface Modification with PEG on Cytotoxicity and Transfection Efficiency. Micro Nano Lett. 2018, 13, 1090–1095. [Google Scholar] [CrossRef]
- Kursa, M.; Walker, G.F.; Roessler, V.; Ogris, M.; Roedl, W.; Kircheis, R.; Wagner, E. Novel Shielded Transferrin−Polyethylene Glycol−Polyethylenimine/DNA Complexes for Systemic Tumor-Targeted Gene Transfer. Bioconjug. Chem. 2003, 14, 222–231. [Google Scholar] [CrossRef]
- Suk, J.S.; Suh, J.; Choy, K.; Lai, S.K.; Fu, J.; Hanes, J. Gene Delivery to Differentiated Neurotypic Cells with RGD and HIV Tat Peptide Functionalized Polymeric Nanoparticles. Biomaterials 2006, 27, 5143–5150. [Google Scholar] [CrossRef]
- Ogris, M.; Walker, G.; Blessing, T.; Kircheis, R.; Wolschek, M.; Wagner, E. Tumor-Targeted Gene Therapy: Strategies for the Preparation of Ligand–Polyethylene Glycol–Polyethylenimine/DNA Complexes. J. Control. Release 2003, 91, 173–181. [Google Scholar] [CrossRef]
- Bahadur, K.C.R.; Thapa, B.; Xu, P. Design of Serum Compatible Tetrary Complexes for Gene Delivery. Macromol. Biosci. 2012, 12, 637–646. [Google Scholar] [CrossRef]
- Belén, L.H.; Rangel-Yagui, C.d.O.; Beltrán Lissabet, J.F.; Effer, B.; Lee-Estevez, M.; Pessoa, A.; Castillo, R.L.; Farías, J.G. From Synthesis to Characterization of Site-Selective PEGylated Proteins. Front. Pharmacol. 2019, 10, 1450. [Google Scholar] [CrossRef]
- Xiong, M.P.; Forrest, M.L.; Karls, A.L.; Kwon, G.S. Biotin-Triggered Release of Poly(Ethylene Glycol)−Avidin from Biotinylated Polyethylenimine Enhances in Vitro Gene Expression. Bioconjug. Chem. 2007, 18, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Arioli, M.; Manfredi, A.; Alongi, J.; Ferruti, P.; Ranucci, E. Highlight on the Mechanism of Linear Polyamidoamine Degradation in Water. Polymers 2020, 12, 1376. [Google Scholar] [CrossRef] [PubMed]
- Almulathanon, A.A.Y.; Ranucci, E.; Ferruti, P.; Garnett, M.C.; Bosquillon, C. Comparison of Gene Transfection and Cytotoxicity Mechanisms of Linear Poly(Amidoamine) and Branched Poly(Ethyleneimine) Polyplexes. Pharm. Res. 2018, 35, 86. [Google Scholar] [CrossRef] [PubMed]
- Franchini, J.; Ranucci, E.; Ferruti, P.; Rossi, M.; Cavalli, R. Synthesis, Physicochemical Properties, and Preliminary Biological Characterizations of a Novel Amphoteric Agmatine-Based Poly(Amidoamine) with RGD-Like Repeating Units. Biomacromolecules 2006, 7, 1215–1222. [Google Scholar] [CrossRef]
- Basak, U.C.; Ghosh, R.; Ghosh, T.; Majumdar, S.; Pakhira, M.; Ghosh, T.; Chatterjee, D.P. Synthesis of ‘Living’ Poly(2-Dimethylaminoethyl Methacrylate) and Stimuli Responsive/Multifunctional Block Copolymers Effective in Fabrication of CdS ‘Smart’ ‘Q-Particles’. Polymer 2018, 155, 27–41. [Google Scholar] [CrossRef]
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st Century: Synthetic Polymers in Drug Delivery Applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Thapa, B.; Narain, R. 1—Mechanism, Current Challenges and New Approaches for Non Viral Gene Delivery. In Polymers and Nanomaterials for Gene Therapy; Narain, R., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 1–27. ISBN 978-0-08-100520-0. [Google Scholar]
- Funhoff, A.M.; van Nostrum, C.F.; Koning, G.A.; Schuurmans-Nieuwenbroek, N.M.E.; Crommelin, D.J.A.; Hennink, W.E. Endosomal Escape of Polymeric Gene Delivery Complexes Is Not Always Enhanced by Polymers Buffering at Low pH. Biomacromolecules 2004, 5, 32–39. [Google Scholar] [CrossRef]
- Luten, J.; Akeroyd, N.; Funhoff, A.; Lok, M.C.; Talsma, H.; Hennink, W.E. Methacrylamide Polymers with Hydrolysis-Sensitive Cationic Side Groups as Degradable Gene Carriers. Bioconjug. Chem. 2006, 17, 1077–1084. [Google Scholar] [CrossRef]
- Hwang, S.J.; Davis, M.E. Cationic polymers for gene delivery: Designs for overcoming barriers to systemic administration. Curr. Opin. Mol. Ther. 2001, 3, 183–191. [Google Scholar]
- Hwang, S.J.; Bellocq, N.C.; Davis, M.E. Effects of Structure of β-Cyclodextrin-Containing Polymers on Gene Delivery. Bioconjug. Chem. 2001, 12, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Dong, X.; Liu, Z.; Liu, G.; Liu, Y. Controllable Singlet Oxygen Generation in Water Based on Cyclodextrin Secondary Assembly for Targeted Photodynamic Therapy. Biomacromolecules 2020, 21, 5369–5379. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dai, X.; Sun, Y.; Liu, Y. Organic Supramolecular Aggregates Based on Water-Soluble Cyclodextrins and Calixarenes. Aggregate 2020, 1, 31–44. [Google Scholar] [CrossRef]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.J.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in Humans from Systemically Administered siRNA via Targeted Nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef]
- Evenou, P.; Rossignol, J.; Pembouong, G.; Gothland, A.; Colesnic, D.; Barbeyron, R.; Rudiuk, S.; Marcelin, A.-G.; Ménand, M.; Baigl, D.; et al. Bridging β-Cyclodextrin Prevents Self-Inclusion, Promotes Supramolecular Polymerization, and Promotes Cooperative Interaction with Nucleic Acids. Angew. Chem. Int. Ed. 2018, 57, 7753–7758. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef]
- Silva, A.O.; Cunha, R.S.; Hotza, D.; Machado, R.A.F. Chitosan as a Matrix of Nanocomposites: A Review on Nanostructures, Processes, Properties, and Applications. Carbohydr. Polym. 2021, 272, 118472. [Google Scholar] [CrossRef]
- Mumper, R.L.; Wang, J.J.; Claspell, J.M.; Rolland, A.P. Novel polymeric condensing carriers for gene delivery. In Proceedings of the International Symposium on Controlled Release of Bioactive Materials, Seattle, WA, USA, 30 July–2 August 1995; Volume 22, pp. 178–179. [Google Scholar]
- Akbuga, J.; Ozbas-Turan, S.; Ekentok, C. Chitosan Nanoparticles in Gene Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 337–351. ISBN 978-3-662-47862-2. [Google Scholar]
- Yu, H.; Chen, X.; Lu, T.; Sun, J.; Tian, H.; Hu, J.; Wang, Y.; Zhang, P.; Jing, X. Poly(l-Lysine)-Graft-Chitosan Copolymers: Synthesis, Characterization, and Gene Transfection Effect. Biomacromolecules 2007, 8, 1425–1435. [Google Scholar] [CrossRef]
- Germershaus, O.; Mao, S.; Sitterberg, J.; Bakowsky, U.; Kissel, T. Gene Delivery Using Chitosan, Trimethyl Chitosan or Polyethylenglycol-Graft-Trimethyl Chitosan Block Copolymers: Establishment of Structure–Activity Relationships In Vitro. J. Control. Release 2008, 125, 145–154. [Google Scholar] [CrossRef]
- Hsueh, Y.-S.; Subramaniam, S.; Tseng, Y.-C.; Chiang, T.-M.; Mestak, O.; Cheng, T.-K.; Kuo, T.-F.; Sivasubramanian, S.; Lin, F.-H.; Shieh, M.-J. In Vitro and in Vivo Assessment of Chitosan Modified Urocanic Acid as Gene Carrier. Mater. Sci. Eng. C 2017, 70, 599–606. [Google Scholar] [CrossRef]
- Wong, K.; Sun, G.; Zhang; Dai, H.; Liu, Y.; He; Leong, K.W. PEI-g-Chitosan, a Novel Gene Delivery System with Transfection Efficiency Comparable to Polyethylenimine in Vitro and after Liver Administration In Vivo. Bioconjug. Chem. 2006, 17, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Thermofisher Scientific. DEAE-Dextran Transfection. Available online: https://www.thermofisher.com/it/en/home/references/gibco-cell-culture-basics/transfection-basics/methods/deae-dextran-transfection.html#:~:text=DEAE-dextran%20is%20a%20polycationic%20derivative%20of%20the%20carbohydrate,used%20to%20increase%20the%20efficiency%20of%20lentiviral%20transduction (accessed on 5 October 2023).
- Hosseinkhani, H.; Azzam, T.; Tabata, Y.; Domb, A. Dextran–Spermine Polycation: An Efficient Nonviral Vector for in Vitro and in Vivo Gene Transfection. Gene Ther. 2004, 11, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Yudovin-Farber, I.; Yanay, C.; Azzam, T.; Linial, M.; Domb, A.J. Quaternary Ammonium Polysaccharides for Gene Delivery. Bioconjug. Chem. 2005, 16, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Guo, Z.; Law, M.-K.; Chen, M. Functionalized PAMAM Constructed Nanosystems for Biomacromolecule Delivery. Biomater. Sci. 2023, 11, 1589–1606. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, L.; Huang, A.Y.-T.; Kao, C.-L.; Peng, L. Poly(Amidoamine) Dendrimers: Covalent and Supramolecular Synthesis. Mater. Today Chem. 2019, 13, 34–48. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechet, J.M.J. Preparation of Polymers with Controlled Molecular Architecture. A New Convergent Approach to Dendritic Macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Kheraldine, H.; Rachid, O.; Habib, A.M.; Al Moustafa, A.-E.; Benter, I.F.; Akhtar, S. Emerging Innate Biological Properties of Nano-Drug Delivery Systems: A Focus on PAMAM Dendrimers and Their Clinical Potential. Adv. Drug Deliv. Rev. 2021, 178, 113908. [Google Scholar] [CrossRef]
- Pavan, G.M.; Albertazzi, L.; Danani, A. Ability to Adapt: Different Generations of PAMAM Dendrimers Show Different Behaviors in Binding siRNA. J. Phys. Chem. B 2010, 114, 2667–2675. [Google Scholar] [CrossRef]
- Kukowska-Latallo, J.F.; Bielinska, A.U.; Johnson, J.; Spindler, R.; Tomalia, D.A.; Baker, J.R. Efficient Transfer of Genetic Material into Mammalian Cells Using Starburst Polyamidoamine Dendrimers. Proc. Natl. Acad. Sci. USA 1996, 93, 4897–4902. [Google Scholar] [CrossRef]
- Bielinska, A.; Chen, C.; Johnson, J.; Baker, J. DNA Complexing with Polyamidoamine Dendrimers: Implications for Transfection. Bioconjug. Chem. 1999, 10, 843–850. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Furini, F.; Casini, A.; Turro, N.J.; Jockusch, S.; Tomalia, D.A.; Messori, L. Formation of Supramolecular Structures between DNA and Starburst Dendrimers Studied by EPR, CD, UV Spectroscopies and Melting Profiles. Macromolecules 2000, 33, 7842–7851. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Chang, H.; Cheng, Y. Surface-Engineered Dendrimers in Gene Delivery. Chem. Rev. 2015, 115, 5274–5300. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.S.; Bae, Y.M.; Kim, J.Y.; Han, J.; Ko, K.S.; Choi, J.S. Amino Acid-Modified Bioreducible Poly(Amidoamine) Dendrimers: Synthesis, Characterization and In Vitro Evaluation. Macromol. Res. 2012, 20, 1156–1162. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Rocchi, P.; Qu, F.; Iovanna, J.L.; Peng, L. Arginine-Terminated Generation 4 PAMAM Dendrimer as an Effective Nanovector for Functional siRNA Delivery in Vitro and in Vivo. Bioconjug. Chem. 2014, 25, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.; Wang, H.; Shao, N.; Chen, Y.; Cheng, Y. Synergistic Effect of Amino Acids Modified on Dendrimer Surface in Gene Delivery. Biomaterials 2014, 35, 9187–9198. [Google Scholar] [CrossRef]
- Thuy, L.T.; Mallick, S.; Choi, J.S. Polyamidoamine (PAMAM) dendrimers modified with short oligopeptides for early endosomal escape and enhanced gene delivery. Int. J. Pharm. 2015, 492, 233–243. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.-S.; Choi, J.S. Basic Amino Acid-Conjugated Polyamidoamine Dendrimers with Enhanced Gene Transfection Efficiency. Macromol. Res. 2014, 22, 500–508. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Kim, M.; Kim, G.; Choi, J.S.; Lee, M. Brain Gene Delivery Using Histidine and Arginine-Modified Dendrimers for Ischemic Stroke Therapy. J. Control. Release 2021, 330, 907–919. [Google Scholar] [CrossRef]
- Yuba, E.; Nakajima, Y.; Tsukamoto, K.; Iwashita, S.; Kojima, C.; Harada, A.; Kono, K. Effect of Unsaturated Alkyl Chains on Transfection Activity of Poly(Amidoamine) Dendron-Bearing Lipids. J. Control. Release 2012, 160, 552–560. [Google Scholar] [CrossRef]
- Santos, J.L.; Oliveira, H.; Pandita, D.; Rodrigues, J.; Pêgo, A.P.; Granja, P.L.; Tomás, H. Functionalization of Poly(Amidoamine) Dendrimers with Hydrophobic Chains for Improved Gene Delivery in Mesenchymal Stem Cells. J. Control. Release 2010, 144, 55–64. [Google Scholar] [CrossRef]
- Biswas, S.; Deshpande, P.P.; Navarro, G.; Dodwadkar, N.S.; Torchilin, V.P. Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery. Biomaterials 2013, 34, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Tsutsumi, T.; Yoshimatsu, A.; Ikeda, H.; Motoyama, K.; Higashi, T.; Hirayama, F.; Uekama, K. Inhibitory Effect of siRNA Complexes with Polyamidoamine Dendrimer/α-Cyclodextrin Conjugate (Generation 3, G3) on Endogenous Gene Expression. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2011, 44, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Kihara, F.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. Effects of Structure of Polyamidoamine Dendrimer on Gene Transfer Efficiency of the Dendrimer Conjugate with α-Cyclodextrin. Bioconjug. Chem. 2002, 13, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Y.-B.; Wang, B.; Lin, R.-Y.; van Dongen, M.; Zurcher, D.M.; Gu, X.-Y.; Banaszak Holl, M.M.; Liu, G.; Qi, R. Efficient in Vitro siRNA Delivery and Intramuscular Gene Silencing Using PEG-Modified PAMAM Dendrimers. Mol. Pharm. 2012, 9, 1812–1821. [Google Scholar] [CrossRef]
- Qi, R.; Gao, Y.; Tang, Y.; He, R.-R.; Liu, T.-L.; He, Y.; Sun, S.; Li, B.-Y.; Li, Y.-B.; Liu, G. PEG-Conjugated PAMAM Dendrimers Mediate Efficient Intramuscular Gene Expression. AAPS J. 2009, 11, 395–405. [Google Scholar] [CrossRef]
- Yuan, Q.; Yeudall, W.A.; Yang, H. PEGylated Polyamidoamine Dendrimers with Bis-Aryl Hydrazone Linkages for Enhanced Gene Delivery. Biomacromolecules 2010, 11, 1940–1947. [Google Scholar] [CrossRef]
- Tariq, I.; Ali, M.Y.; Sohail, M.F.; Amin, M.U.; Ali, S.; Bukhari, N.I.; Raza, A.; Pinnapireddy, S.R.; Schäfer, J.; Bakowsky, U. Lipodendriplexes Mediated Enhanced Gene Delivery: A Cellular to Pre-Clinical Investigation. Sci. Rep. 2020, 10, 21446. [Google Scholar] [CrossRef]
- Chang, H.; Wang, H.; Shao, N.; Wang, M.; Wang, X.; Cheng, Y. Surface-Engineered Dendrimers with a Diaminododecane Core Achieve Efficient Gene Transfection and Low Cytotoxicity. Bioconjug. Chem. 2014, 25, 342–350. [Google Scholar] [CrossRef]
- Wang, M.; Liu, H.; Li, L.; Cheng, Y. A Fluorinated Dendrimer Achieves Excellent Gene Transfection Efficacy at Extremely Low Nitrogen to Phosphorus Ratios. Nat. Commun. 2014, 5, 3053. [Google Scholar] [CrossRef]
- Chang, H.; Zhang, J.; Wang, H.; Lv, J.; Cheng, Y. A Combination of Guanidyl and Phenyl Groups on a Dendrimer Enables Efficient siRNA and DNA Delivery. Biomacromolecules 2017, 18, 2371–2378. [Google Scholar] [CrossRef]
- Fang, H.; Guo, Z.; Lin, L.; Chen, J.; Sun, P.; Wu, J.; Xu, C.; Tian, H.; Chen, X. Molecular Strings Significantly Improved the Gene Transfection Efficiency of Polycations. J. Am. Chem. Soc. 2018, 140, 11992–12000. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Gorman, C.B.; Menegatti, S. DendriPeps: Expanding Dendrimer Functionality by Hybridizing Poly(Amidoamine) (PAMAM) Scaffolds with Peptide Segments. Macromol. Rapid Commun. 2019, 40, 1900325. [Google Scholar] [CrossRef] [PubMed]
- Joubert, F.; Munson, M.J.; Sabirsh, A.; England, R.M.; Hemmerling, M.; Alexander, C.; Ashford, M.B. Precise and Systematic End Group Chemistry Modifications on PAMAM and Poly(l-Lysine) Dendrimers to Improve Cytosolic Delivery of mRNA. J. Control. Release 2023, 356, 580–594. [Google Scholar] [CrossRef]
- Mishra, V.; Yadav, N.; Saraogi, G.K.; Tambuwala, M.M.; Giri, N. Dendrimer Based Nanoarchitectures in Diabetes Management: An Overview. Curr. Pharm. Des. 2019, 25, 2569–2583. [Google Scholar] [CrossRef]
- Salvi, L.; Dubey, C.; Sharma, K.; Nagar, D.; Meghani, M.; Goyal, S.; Nagar, J.; Sharma, A. A Synthesis, Properties and Application as a Possible Drug Delivery Systems Dendrimers—A Review. Asian J. Pharm. Res. Dev. 2020, 8, 107–113. [Google Scholar] [CrossRef]
- Kabanov, V.A.; Sergeyev, V.G.; Pyshkina, O.A.; Zinchenko, A.A.; Zezin, A.B.; Joosten, J.G.H.; Brackman, J.; Yoshikawa, K. Interpolyelectrolyte Complexes Formed by DNA and Astramol Poly(Propylene Imine) Dendrimers. Macromolecules 2000, 33, 9587–9593. [Google Scholar] [CrossRef]
- Hashemi, M.; Parhiz, H.; Mokhtarzadeh, A.; Tabatabai, S.M.; Farzad, S.A.; Shirvan, H.R.; Ramezani, M. Preparation of Effective and Safe Gene Carriers by Grafting Alkyl Chains to Generation 5 Polypropyleneimine. AAPS PharmSciTech 2015, 16, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Baek, J.; Zhe Bai, C.; Park, J. Arginine-Conjugated Polypropylenimine Dendrimer as a Non-Toxic and Efficient Gene Delivery Carrier. Biomaterials 2007, 28, 2061–2067. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, Y.H.; Park, S.-H.; Yamaguchi, K.; Kim, K. Novel Pseudorotaxane-Terminated Dendrimers: Supramolecular Modification of Dendrimer Periphery. Angew. Chem. Int. Ed. 2001, 40, 746–749. [Google Scholar] [CrossRef]
- Malkoch, M.; Malmström, E.; Nyström, A.M. 6.04—Dendrimers: Properties and Applications. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 113–176. ISBN 978-0-08-087862-1. [Google Scholar]
- Posnett, D.N.; McGrath, H.; Tam, J.P. A Novel Method for Producing Anti-Peptide Antibodies. Production of Site-Specific Antibodies to the T Cell Antigen Receptor Beta-Chain. J. Biol. Chem. 1988, 263, 1719–1725. [Google Scholar] [CrossRef]
- Grandjean, C.; Rommens, C.; Gras-masse, H.; Melnyk, O. One-Pot Synthesis of Antigen-Bearing, Lysine-Based Cluster Mannosides Using Two Orthogonal Chemoselective Ligation Reactions. Angew. Chem. 2000, 39, 1068–1072. [Google Scholar] [CrossRef]
- Melnyk, O.; Fruchart, J.-S.; Grandjean, C.; Gras-Masse, H. Tartric Acid-Based Linker for the Solid-Phase Synthesis of C-Terminal Peptide α-Oxo Aldehydes. J. Org. Chem. 2001, 66, 4153–4160. [Google Scholar] [CrossRef] [PubMed]
- Kantchev, E.A.B.; Chang, C.-C.; Cheng, S.-F.; Roche, A.-C.; Chang, D.-K. Direct Solid-Phase Synthesis and Fluorescence Labeling of Large, Monodisperse Mannosylated Dendrons in a Peptide Synthesizer. Org. Biomol. Chem. 2008, 6, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Kantchev, E.A.B.; Chang, C.-C.; Chang, D.-K. Direct Fmoc/Tert-Bu Solid Phase Synthesis of Octamannosyl Polylysine Dendrimer–Peptide Conjugates. Pept. Sci. 2006, 84, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Driffield, M.; Goodall, D.M.; Klute, A.S.; Smith, D.K.; Wilson, K. Synthesis and Characterization of Silica-Supported l-Lysine-Based Dendritic Branches. Langmuir 2002, 18, 8660–8665. [Google Scholar] [CrossRef]
- Chapman, T.M.; Hillyer, G.L.; Mahan, E.J.; Shaffer, K.A. Hydraamphiphiles: Novel Linear Dendritic Block Copolymer Surfactants. J. Am. Chem. Soc. 1994, 116, 11195–11196. [Google Scholar] [CrossRef]
- Grandjean, C.; Rommens, C.; Gras-Masse, H.; Melnyk, O. Convergent Synthesis of Fluorescein-Labelled Lysine-Based Cluster Glycosides. Tetrahedron Lett. 1999, 40, 7235–7238. [Google Scholar] [CrossRef]
- Hirst, A.R.; Smith, D.K.; Feiters, M.C.; Geurts, H.P.M.; Wright, A.C. Two-Component Dendritic Gels: Easily Tunable Materials. J. Am. Chem. Soc. 2003, 125, 9010–9011. [Google Scholar] [CrossRef]
- Okuda, T.; Kidoaki, S.; Ohsaki, M.; Koyama, Y.; Yoshikawa, K.; Niidome, T.; Aoyagi, H. Time-Dependent Complex Formation of Dendritic Poly(L-Lysine) with Plasmid DNA and Correlation with in Vitro Transfection Efficiencies. Org. Biomol. Chem. 2003, 1, 1270–1273. [Google Scholar] [CrossRef]
- Yamagata, M.; Kawano, T.; Shiba, K.; Mori, T.; Katayama, Y.; Niidome, T. Structural Advantage of Dendritic Poly(l-Lysine) for Gene Delivery into Cells. Bioorg. Med. Chem. 2007, 15, 526–532. [Google Scholar] [CrossRef]
- Sheveleva, N.N.; Markelov, D.A.; Vovk, M.A.; Mikhailova, M.E.; Tarasenko, I.I.; Tolstoy, P.M.; Neelov, I.M.; Lähderanta, E. Lysine-Based Dendrimer with Double Arginine Residues. RSC Adv. 2019, 9, 18018–18026. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.A.; Kiselev, A.V.; Tarasenko, I.I.; Il’ina, P.L.; Pankova, G.A.; Il’ina, I.E.; Baranov, V.C.; Vlasov, G.P. Hyperbranched Polylysines Modified with Histidine and Arginine: The Optimization of Their DNA Compacting and Endosomolytic Properties. Russ. J. Bioorg. Chem. 2009, 35, 437–445. [Google Scholar] [CrossRef]
- Kaiser, T.; Frey, H. Hyperbranched Polymer Architectures: From Flory’s AB(f-1) Polycondensates to Controlled Structures. Polymer 2020, 211, 123113. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.D.; Voegtle, F. “Cascade”- and “Nonskid-Chain-like” Syntheses of Molecular Cavity Topologies. Synthesis 1978, 1978, 155–158. [Google Scholar] [CrossRef]
- Dendrimers. Available online: https://en.wikipedia.org/wiki/Dendrimer (accessed on 26 September 2023).
- Walter, M.V.; Malkoch, M. Simplifying the Synthesis of Dendrimers: Accelerated Approaches. Chem. Soc. Rev. 2012, 41, 4593–4609. [Google Scholar] [CrossRef]
- Dimethylolpropionic. Available online: https://en.wikipedia.org/wiki/Dimethylolpropionic_acid (accessed on 26 September 2023).
- Carlmark, A.; Malmström, E.; Malkoch, M. Dendritic Architectures Based on Bis-MPA: Functional Polymeric Scaffolds for Application-Driven Research. Chem. Soc. Rev. 2013, 42, 5858–5879. [Google Scholar] [CrossRef]
- Patel, P.; Patel, V.; Patel, P.M. Synthetic Strategy of Dendrimers: A Review. J. Indian Chem. Soc. 2022, 99, 100514. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Pineda-Castañeda, H.M.; Rivera-Monroy, Z.J.; Maldonado, M. Copper(I)-Catalyzed Alkyne–Azide Cycloaddition (CuAAC) “Click” Reaction: A Powerful Tool for Functionalizing Polyhydroxylated Platforms. ACS Omega 2023, 8, 3650–3666. [Google Scholar] [CrossRef]
- Ihre, H.; Padilla De Jesús, O.L.; Fréchet, J.M. Fast and convenient divergent synthesis of aliphatic ester dendrimers by anhydride coupling. J. Am. Chem. Soc. 2001, 123, 5908–5917. [Google Scholar] [CrossRef]
- Malkoch, M.; Malmström, E.; Hult, A. Rapid and Efficient Synthesis of Aliphatic Ester Dendrons and Dendrimers. Macromolecules 2002, 35, 8307–8314. [Google Scholar] [CrossRef]
- Ihre, H.; Hult, A.; Söderlind, E. Synthesis, Characterization, and 1H NMR Self-Diffusion Studies of Dendritic Aliphatic Polyesters Based on 2,2-Bis(Hydroxymethyl)Propionic Acid and 1,1,1-Tris(Hydroxyphenyl)Ethane. J. Am. Chem. Soc. 1996, 118, 6388–6395. [Google Scholar] [CrossRef]
- Wooley, K.L.; Hawker, C.J.; Fréchet, J.M.J. A “Branched-Monomer Approach” for the Rapid Synthesis of Dendimers. Angew. Chem. Int. Ed. Engl. 1994, 33, 82–85. [Google Scholar] [CrossRef]
- Xu, Z.; Kahr, M.; Walker, K.L.; Wilkins, C.L.; Moore, J.S. Phenylacetylene Dendrimers by the Divergent, Convergent, and Double-Stage Convergent Methods. J. Am. Chem. Soc. 1994, 116, 4537–4550. [Google Scholar] [CrossRef]
- Ihre, H.; Hult, A.; Fréchet, J.M.J.; Gitsov, I. Double-Stage Convergent Approach for the Synthesis of Functionalized Dendritic Aliphatic Polyesters Based on 2,2-Bis(Hydroxymethyl)Propionic Acid. Macromolecules 1998, 31, 4061–4068. [Google Scholar] [CrossRef]
- Vestberg, R.; Nyström, A.; Lindgren, M.; Malmström, E.; Hult, A. Porphyrin-Cored 2,2-Bis(Methylol)Propionic Acid Dendrimers. Chem. Mater. 2004, 16, 2794–2804. [Google Scholar] [CrossRef]
- Goodwin, A.P.; Lam, S.S.; Fréchet, J.M.J. Rapid, Efficient Synthesis of Heterobifunctional Biodegradable Dendrimers. J. Am. Chem. Soc. 2007, 129, 6994–6995. [Google Scholar] [CrossRef]
- Guillaudeu, S.J.; Fox, M.E.; Haidar, Y.M.; Dy, E.E.; Szoka, F.C.; Fréchet, J.M.J. PEGylated Dendrimers with Core Functionality for Biological Applications. Bioconjug. Chem. 2008, 19, 461–469. [Google Scholar] [CrossRef]
- Wu, P.; Feldman, A.K.; Nugent, A.K.; Hawker, C.J.; Scheel, A.; Voit, B.; Pyun, J.; Fréchet, J.M.J.; Sharpless, K.B.; Fokin, V.V. Efficiency and Fidelity in a Click-Chemistry Route to Triazole Dendrimers by the Copper(I)-Catalyzed Ligation of Azides and Alkynes. Angew. Chem. Int. Ed. 2004, 43, 3928–3932. [Google Scholar] [CrossRef]
- Antoni, P.; Nyström, D.; Hawker, C.J.; Hult, A.; Malkoch, M. A Chemoselective Approach for the Accelerated Synthesis of Well-Defined Dendritic Architectures. Chem. Commun. 2007, 22, 2249–2251. [Google Scholar] [CrossRef]
- Carlmark, A.; Hawker, C.; Hult, A.; Malkoch, M. New Methodologies in the Construction of Dendritic Materials. Chem. Soc. Rev. 2009, 38, 352–362. [Google Scholar] [CrossRef] [PubMed]
- García-Gallego, S.; Stenström, P.; Mesa-Antunez, P.; Zhang, Y.; Malkoch, M. Synthesis of Heterofunctional Polyester Dendrimers with Internal and External Functionalities as Versatile Multipurpose Platforms. Biomacromolecules 2020, 21, 4273–4279. [Google Scholar] [CrossRef] [PubMed]
- Gillies, E.R.; Fréchet, J.M.J. Designing Macromolecules for Therapeutic Applications: Polyester Dendrimer-Poly(Ethylene Oxide) “Bow-Tie” Hybrids with Tunable Molecular Weight and Architecture. J. Am. Chem. Soc. 2002, 124, 14137–14146. [Google Scholar] [CrossRef] [PubMed]
- Gitsov, I.; Wooley, K.; Frechet, J. Novel polyether copolymers consisting of linear and dendritic blocks. Angew. Chem.-Int. Ed. Engl. 1992, 31, 1200–1202. [Google Scholar] [CrossRef]
- Hawker, C.J. Dendritic and Hyperbranched Macromolecules—Precisely Controlled Macromolecular Architectures. In Macromolecular Architectures; Hilborn, J.G., Dubois, P., Hawker, C.J., Hedrick, J.L., Hilborn, J.G., Jérôme, R., Kiefer, J., Labadie, J.W., Mecerreyes, D., Volksen, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 113–160. ISBN 978-3-540-49196-5. [Google Scholar]
- Gitsov, I.; Wooley, K.L.; Hawker, C.J.; Ivanova, P.T.; Frechet, J.M.J. Synthesis and Properties of Novel Linear-Dendritic Block Copolymers. Reactivity of Dendritic Macromolecules toward Linear Polymers. Macromolecules 1993, 26, 5621–5627. [Google Scholar] [CrossRef]
- Sun, H.; Haque, F.M.; Zhang, Y.; Commisso, A.; Mohamed, M.A.; Tsianou, M.; Cui, H.; Grayson, S.M.; Cheng, C. Linear-Dendritic Alternating Copolymers. Angew. Chem. Int. Ed. 2019, 58, 10572–10576. [Google Scholar] [CrossRef]
- Frauenrath, H. Dendronized Polymers—Building a New Bridge from Molecules to Nanoscopic Objects. Prog. Polym. Sci. 2005, 30, 325–384. [Google Scholar] [CrossRef]
- Zhang, A.; Shu, L.; Bo, Z.; Schlüter, A.D. Dendronized Polymers: Recent Progress in Synthesis. Macromol. Chem. Phys. 2003, 204, 328–339. [Google Scholar] [CrossRef]
- Trollsås, M.; Hedrick, J.L.; Mecerreyes, D.; Dubois, P.; Jérôme, R.; Ihre, H.; Hult, A. Versatile and Controlled Synthesis of Star and Branched Macromolecules by Dentritic Initiation. Macromolecules 1997, 30, 8508–8511. [Google Scholar] [CrossRef]
- Atthoff, B.; Trollsås, M.; Claesson, H.; Hedrick, J.L. Poly(Lactides) with Controlled Molecular Architecture Initiated from Hydroxyl Functional Dendrimers and the Effect on the Hydrodynamic Volume. Macromol. Chem. Phys. 1999, 200, 1333–1339. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Wu, Z.; Lundberg, P.; Malkoch, M.; Nyström, A.M. Hyperbranched Copolymer Micelles as Delivery Vehicles of Doxorubicin in Breast Cancer Cells. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 280–288. [Google Scholar] [CrossRef]
- del Barrio, J.; Oriol, L.; Alcalá, R.; Sánchez, C. Azobenzene-Containing Linear−Dendritic Diblock Copolymers by Click Chemistry: Synthesis, Characterization, Morphological Study, and Photoinduction of Optical Anisotropy. Macromolecules 2009, 42, 5752–5760. [Google Scholar] [CrossRef]
- Altin, H.; Kosif, I.; Sanyal, R. Fabrication of “Clickable” Hydrogels via Dendron−Polymer Conjugates. Macromolecules 2010, 43, 3801–3808. [Google Scholar] [CrossRef]
- Montañez, M.I.; Hed, Y.; Utsel, S.; Ropponen, J.; Malmström, E.; Wågberg, L.; Hult, A.; Malkoch, M. Bifunctional Dendronized Cellulose Surfaces as Biosensors. Biomacromolecules 2011, 12, 2114–2125. [Google Scholar] [CrossRef]
- Liu, P. Hyperbranched Aliphatic Polyester Grafted Silica Nanoparticles by a Facile One-Pot Method. Surf. Rev. Lett. 2005, 12, 619–622. [Google Scholar] [CrossRef]
- Liu, P. Hyperbranched Aliphatic Polyester Grafted Attapulgite via a Melt Polycondensation Process. Appl. Clay Sci. 2007, 35, 11–16. [Google Scholar] [CrossRef]
- Liu, p.; Zhang, L. Hyperbranched aliphatic polyester modified activated carbon particles with homogenized surface groups. Surf. Rev. Lett. 2007, 14, 1025–1032. [Google Scholar] [CrossRef]
- Benhabbour, S.R.; Liu, L.; Sheardown, H.; Adronov, A. Protein Resistance of Surfaces Prepared by Chemisorption of Monothiolated Poly(Ethylene Glycol) to Gold and Dendronization with Aliphatic Polyester Dendrons: Effect of Hydrophilic Dendrons. Macromolecules 2008, 41, 2567–2576. [Google Scholar] [CrossRef]
- Benhabbour, S.R.; Sheardown, H.; Adronov, A. Protein Resistance of PEG-Functionalized Dendronized Surfaces: Effect of PEG Molecular Weight and Dendron Generation. Macromolecules 2008, 41, 4817–4823. [Google Scholar] [CrossRef]
- Ledin, P.A.; Friscourt, F.; Guo, J.; Boons, G.-J. Convergent Assembly and Surface Modification of Multifunctional Dendrimers by Three Consecutive Click Reactions. Chem.—A Eur. J. 2011, 17, 839–846. [Google Scholar] [CrossRef]
- Lo Conte, M.; Robb, M.J.; Hed, Y.; Marra, A.; Malkoch, M.; Hawker, C.J.; Dondoni, A. Exhaustive Glycosylation, PEGylation, and Glutathionylation of a [G4]-ene(48) Dendrimer via Photoinduced Thiolene Coupling. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4468–4475. [Google Scholar] [CrossRef]
- Padilla De Jesús, O.L.; Ihre, H.R.; Gagne, L.; Fréchet, J.M.; Szoka, F.C., Jr. Polyester dendritic systems for drug delivery applications: In Vitro and in vivo evaluation. Bioconjug. Chem. 2002, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Gillies, E.R.; Fox, M.E.; Guillaudeu, S.J.; Fréchet, J.M.J.; Dy, E.E.; Szoka, F.C. A Single Dose of Doxorubicin-Functionalized Bow-Tie Dendrimer Cures Mice Bearing C-26 Colon Carcinomas. Proc. Natl. Acad. Sci. USA 2006, 103, 16649–16654. [Google Scholar] [CrossRef] [PubMed]
- Antoni, P.; Robb, M.J.; Campos, L.; Montanez, M.; Hult, A.; Malmström, E.; Malkoch, M.; Hawker, C.J. Pushing the Limits for Thiol−Ene and CuAAC Reactions: Synthesis of a 6th Generation Dendrimer in a Single Day. Macromolecules 2010, 43, 6625–6631. [Google Scholar] [CrossRef]
- Montañez, M.I.; Campos, L.M.; Antoni, P.; Hed, Y.; Walter, M.V.; Krull, B.T.; Khan, A.; Hult, A.; Hawker, C.J.; Malkoch, M. Accelerated Growth of Dendrimers via Thiol−Ene and Esterification Reactions. Macromolecules 2010, 43, 6004–6013. [Google Scholar] [CrossRef]
- Malkoch, M.; Schleicher, K.; Drockenmuller, E.; Hawker, C.J.; Russell, T.P.; Wu, P.; Fokin, V.V. Structurally Diverse Dendritic Libraries: A Highly Efficient Functionalization Approach Using Click Chemistry. Macromolecules 2005, 38, 3663–3678. [Google Scholar] [CrossRef]
- Wu, P.; Malkoch, M.; Hunt, J.N.; Vestberg, R.; Kaltgrad, E.; Finn, M.G.; Fokin, V.V.; Sharpless, K.B.; Hawker, C.J. Multivalent, Bifunctional Dendrimers Prepared by Click Chemistry. Chem. Commun. 2005, 46, 5775–5777. [Google Scholar] [CrossRef]
- Namata, F.; Sanz del Olmo, N.; Molina, N.; Malkoch, M. Synthesis and Characterization of Amino-Functional Polyester Dendrimers Based on Bis-MPA with Enhanced Hydrolytic Stability and Inherent Antibacterial Properties. Biomacromolecules 2023, 24, 858–867. [Google Scholar] [CrossRef]
- Hao, M.; Zhang, L.; Chen, P. Membrane Internalization Mechanisms and Design Strategies of Arginine-Rich Cell-Penetrating Peptides. Int. J. Mol. Sci. 2022, 23, 9038. [Google Scholar] [CrossRef]
- Liu, B.R.; Chiou, S.-H.; Huang, Y.-W.; Lee, H.-J. Bio-Membrane Internalization Mechanisms of Arginine-Rich Cell-Penetrating Peptides in Various Species. Membranes 2022, 12, 88. [Google Scholar] [CrossRef]
- Ryu, Y.C.; Kim, K.A.; Kim, B.C.; Wang, H.-M.D.; Hwang, B.H. Novel Fusion Peptide-mediated siRNA Delivery Using Self-assembled Nanocomplex. J. Nanobiotechnol. 2021, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Morris, V.B.; Labhasetwar, V. Effectiveness of Small Interfering RNA Delivery via Arginine-Rich Polyethylenimine-Based Polyplex in Metastatic and Doxorubicin-Resistant Breast Cancer Cells. J. Pharmacol. Exp. Ther. 2019, 370, 902. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Lin, H.; Yu, Y.; Shi, L.; Tu, J. Precisely Defined Polymers for Efficient Gene Delivery. Top. Curr. Chem. 2018, 376, 2. [Google Scholar] [CrossRef] [PubMed]
- Strašák, T.; Malý, J.; Wróbel, D.; Malý, M.; Herma, R.; Čermák, J.; Müllerová, M.; Št′astná, L.Č.; Cuřínová, P. Phosphonium Carbosilane Dendrimers for Biomedical Applications—Synthesis, Characterization and Cytotoxicity Evaluation. RSC Adv. 2017, 7, 18724–18744. [Google Scholar] [CrossRef]
- CD Bioparticles. Available online: https://www.cd-bioparticles.net/p/10472/dmpa-g5-carboxyl-oh (accessed on 24 October 2023).














































| Strategies | Drawbacks | Ref. | ||
|---|---|---|---|---|
| Conventional Approaches | DG | Defective Dendrimers ⇑ Synthetic time ⇑ Excess of reagents Laborious purification | [161] | |
| CG | ⇑ Time ⇑ Excess of reagents Laborious purification | |||
| Revisited Approach | Accelerate Synthesis | Hyper monomer (HM) | Multistage synthesis of HM ⇓ Yields | |
| Two-Stage CG (TSCG) | Laborious and multistage synthesis of low-generation dendrons and dendrimers (hypercore) | |||
| Double exponential grow (DEG) | ⇓ Yields for high-generation dendrimers | |||
| Advantageous Accelerate Synthesis | Advantages | Ref. | ||
| Click Chemistry | ⇑ Thermodynamic thrust | CuAAC | Efficiency Selectivity ⇑ Yields ⇓ Steps | [162,163] |
| Crucial Points | Considerations |
|---|---|
| Careful selection of starting monomers | The speed of synthesis and the physicochemical characteristics of the final dendrimer depend on the molecular structure of monomers and the number and nature of their functional groups. |
| Careful design of the number of reactions to carry out for realizing the growth of generations | In conventional strategies, activation is a necessary step, while its elimination would decrease the number of overall reactions. |
| Possible use of one-pot reactions | It is very desirable because if the number of synthetic and purification steps of the final products were reduced, the overall time of the synthesis would be shortened. |
| Approach | Procedure | Disadvantages | Polymer-Dendrimer System | Ref. |
|---|---|---|---|---|
| Graft-from (GF) | Function A of b-HMPA was linked to a polymeric chain via an ester or amide bond. The dendrimer structures were then built in a diverging way using the two terminal hydroxyl groups (B) | Risk of creating defective structures | Linear | [182] |
| Graft-to (GT) | Function A of b-HMPA was used to bind preformed dendrons to the polymeric chain, having functions capable of reacting with the carboxylic one | Risk of incomplete functionalization | [182,183] | |
| Macromonomer (MM) | Polymerization of dendritic MMs | Difficult synthesis of the MM ⇓ Polymerization yields * | [182,183] | |
| Core-first (CF) | A dendrimer plays the role of the macro-initiator of the polymerization | Difficult purification and characterization when incomplete functionalization and defective structures occur | Star-like | [184,185] |
| Arm-first (AF) | A dendrimer plays the role of support for the attachment of preformed polymers | [186] | ||
| Divergent grow | Benzylidene anhydride (2) was coupled via ester linkage to mono-, di-, and tetra- hydroxylated PEG polymers. A divergent growth of dendrons was performed, starting from the functionalized PEGs. | N.R. | Dendritic-linear block co-polymers  | [164] |
| DSCM + Click chemistry | Azo-dendrons based on b-HMPA were prepared by DSCM and were attached to an alkyne-functionalized PEG by a Huisgen’s 1,3-dipolar cycloaddition (Click chemistry) | [187] | ||
| CuAAC reaction | Dendrons derived from anhydride (3) functionalized with terminal alkyne residues were connected to PEG polymers activated with azide groups | [188] |
| Age Group | Core/Hyper Core | Synthetic Strategy | Generation | OH | N+ | Peripheral Functionalization | Amino Acids | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1st | t-HMPO | Convergent grow | G4 | 48 | 48 | One type of amino acid (AA) for dendrimer | Gly | [26] |
| 48 | MGly | |||||||
| 48 | GABA | |||||||
| 48 | MGABA | |||||||
| 96 | Lys | |||||||
| 96 | His | |||||||
| G4OH | TSCG * | G5 | NED | 96 | One or two types of AA for dendrimer | Gly | ||
| 96 | MGly | |||||||
| 96 | GABA | |||||||
| 192 | Lys | |||||||
| 192 | His | |||||||
| 192 | Lys + His | |||||||
| G6 | 192 | One type of AA for dendrimer | Gly | |||||
| 192 | GABA | |||||||
| 384 | Lys | |||||||
| 2nd | t-HMPO | Convergent grow | G4 | 48 | 72 | One or two types of AA for dendrimer | Arg | [13] |
| 58 | ArgGly | |||||||
| 96 | Arg + Lys | |||||||
| 57 | MeOTyr + Arg | |||||||
| G4OH | TSCG ** | G5 | 96 | 132 | Arg | |||
| 104 | ArgGly | |||||||
| 136 | Arg + Lys | |||||||
| 3rd | t-HMPO | Convergent grow | G4 | 48 | 81 | Four types of AA for dendrimer | DMG + MGly + Arg + Lys | [7] |
| 79 | ||||||||
| 4th | t-HMPO-MS | Convergent grow | G2 | 8 | 16 | One type of AA for dendrimer | Arg | [1] |
| 8 | ||||||||
| TSCG *** | G3 | 16 | 28 | Two types of AA for dendrimer | Arg + Lys | |||
| 24 | ||||||||
| Pentaerythritol-MS | TSCG *** | G3 | 24 | 37 | Four types of AA for dendrimer | DMG + MGly + Arg + Lys |
| Structure | Commercial Name | Features |
|---|---|---|
 | DMPA-G1-TMP-Acetylene | MW: 1311 g/mol Core: Trimethylol Propane Number of Surface Groups: 6 Generation: 1 |
 | DMPA-G1-TMP-Azide | MW: 1317 g/mol Core: Trimethylol Propane Number of Surface Groups: 6 Generation: 1 |
 | DMPA-G1-TMP-Carboxyl | MW: 1083 g/mol Core: Trimethylol Propane Number of Surface Groups: 2 Generation: 1 |
 | DMPA-G1-TMP-NHBOC | Core: Trimethylol Propane Number of Surface Groups: 6 Generation: 1 |
 | DMPA-G1-TMP-OH | MW: 482.52 g/mol Core: Trimethylol Propane Number of Surface Groups: 6 Generation: 1 |
 | DMPA-G1-TMP-RAFT3 | Core: Trimethylol Propane Number of Surface Groups: 6 Generation: 1 |
 | DMPA-G2-TMP-Acetylene | MW: 2837 g/mol Core: Trimethylol Propane Number of Surface Groups: 12 Generation: 2 |
 | DMPA-G2-TMP-Ammonium | MW: 2044 g/mol Core: Trimethylol Propane Number of Surface Groups: 12 Generation: 2 |
 | DMPA-G2-TMP-Azide | MW: 2849 g/mol Core: Trimethylol Propane Number of Surface Groups: 12 Generation: 2 |
 | DMPA-G2-TMP-Carboxyl | MW: 11,989 g/mol Core: Trimethylol Propane Number of Surface Groups: 12 Generation: 2 |
 | DMPA-G2-TMP-NHBOC | MW: 3234 g/mol Core: Trimethylol Propane Number of Surface Groups: 12 Generation: 2 |
 | DMPA-G2-TMP-OH | MW: 1179.21 g/mol Core: Trimethylol Propane Number of Surface Groups: 12 Generation: 2 |
 | DMPA-G3-TMP-Acetylene | MW: 5888 g/mol Core: Trimethylol Propane Number of Surface Groups: 24 Generation: 3 |
 | DMPA-G3-TMP-Ammonium | MW: 4303 g/mol Core: Trimethylol Propane Number of Surface Groups: 24 Generation: 3 |
 | DMPA-G3-TMP-Azide | MW: 5912 g/mol Core: Trimethylol Propane Number of Surface Groups: 24 Generation: 3 |
 | DMPA-G3-TMP-Carboxyl | MW: 4974 g/mol Core: Trimethylol Propane Number of Surface Groups: 8 Generation: 3 |
 | DMPA-G3-TMP-OH | MW: 2572.59 g/mol Core: Trimethylol Propane Number of Surface Groups: 24 Generation: 3 |
 | DMPA-G4-TMP-Acetylene | MW: 11,989 g/mol Core: Trimethylol Propane Number of Surface Groups: 48 Generation: 4 |
 | DMPA-G4-TMP-Ammonium | MW: 8818 g/mol Core: Trimethylol Propane Number of Surface Groups: 48 Generation: 4 |
 | DMPA-G4-TMP-Azide | MW: 12,038.98 g/mol Core: Trimethylol Propane Number of Surface Groups: 48 Generation: 4 |
 | DMPA-G4-TMP-Carboxyl | MW: 10,163 g/mol Core: Trimethylol Propane Number of Surface Groups: 48 Generation: 4 |
 | DMPA-G4-TMP-NHBOC | MW: 13,577 g/mol Core: Trimethylol Propane Number of Surface Groups: 48 Generation: 4 |
 | DMPA-G4-TMP-OH | MW: 5357.4 g/mol Core: Trimethylol Propane Number of Surface Groups: 48 Generation: 4 |
 | DMPA-G5-TMP-Acetylene | MW: 24,193 g/mol Core: Trimethylol Propane Number of Surface Groups: 96 Generation: 5 |
 | DMPA-G5-TMP-Azide | MW: 24,292 g/mol Core: Trimethylol Propane Number of Surface Groups: 96 Generation: 5 |
 | DMPA-G5-TMP-Carboxyl | MW: 20,540 g/mol Core: Trimethylol Propane Number of Surface Groups: 96 Generation: 5 |
 | DMPA-G5-TMP-OH | MW: 10,933 g/mol Core: Trimethylol Propane Number of Surface Groups: 96 Generation: 5 |
 | Hyperbranched G2-PEG 10k-OH | MW: 10,698.64 g/mol Core: PEG 10,000 Number of Surface Groups: 8 Generation: 2 |
 | Hyperbranched G3-PEG 10k-OH | MW: 11,643.56 g/mol Core: PEG 10,000 Number of Surface Groups: 16 Generation: 3 |
 | Hyperbranched G4-PEG 20k-OH | MW: 23,545.39 g/mol Core: PEG 20,000 Number of Surface Groups: 32 Generation: 4 |
 | Hyperbranched G4-PEG 6k-OH | MW: 9460.49 g/mol Core: PEG 6000 Number of Surface Groups: 32 Generation: 4 |
 | Hyperbranched G6-PEG 10k-OH | MW: 24,567.96 g/mol Core: PEG 10,000 Number of Surface Groups: 128 Generation: 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S. Cationic Materials for Gene Therapy: A Look Back to the Birth and Development of 2,2-Bis-(hydroxymethyl)Propanoic Acid-Based Dendrimer Scaffolds. Int. J. Mol. Sci. 2023, 24, 16006. https://doi.org/10.3390/ijms242116006
Alfei S. Cationic Materials for Gene Therapy: A Look Back to the Birth and Development of 2,2-Bis-(hydroxymethyl)Propanoic Acid-Based Dendrimer Scaffolds. International Journal of Molecular Sciences. 2023; 24(21):16006. https://doi.org/10.3390/ijms242116006
Chicago/Turabian StyleAlfei, Silvana. 2023. "Cationic Materials for Gene Therapy: A Look Back to the Birth and Development of 2,2-Bis-(hydroxymethyl)Propanoic Acid-Based Dendrimer Scaffolds" International Journal of Molecular Sciences 24, no. 21: 16006. https://doi.org/10.3390/ijms242116006