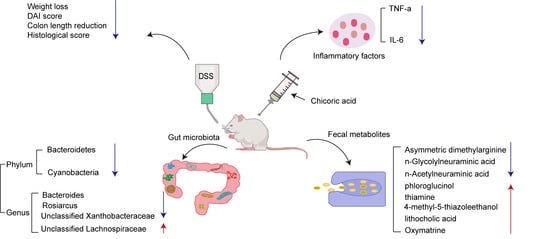
Chicoric Acid Effectively Mitigated Dextran Sulfate Sodium (DSS)-Induced Colitis in BALB/c Mice by Modulating the Gut Microbiota and Fecal Metabolites
Abstract
:1. Introduction
2. Results
2.1. Supplementation with CA Alleviated DSS-Induced Colitis in Mice
2.2. Supplementation with CA Attenuated DSS-Induced Colon Shortening and Colonic Histological Damage
2.3. Supplementation with CA Reduced the Levels of Inflammatory Factors in Mouse Serum
2.4. Supplementation with CA Regulated Gut Microbiota
2.5. Supplementation with CA Altered Fecal Metabolites in Colitis Mice
2.6. Correlation Analysis between Gut Microbiota, Fecal Differential Metabolites, and Host Phenotypes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Experimental Design
4.3. Disease Activity Index (DAI) Assessment
4.4. Colonic Hematoxylin and Eosin (H&E) Staining and Histopathological Analysis
4.5. Inflammatory Cytokine Assay
4.6. 16S rRNA DNA Sequencing
4.7. Untargeted Metabolomics Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Chicoric acid |
| DSS | Dextran sulfate sodium |
| DAI | Disease activity index |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin- 6 |
| IBD | Inflammatory bowel disease |
| UC | Ulcerative colitis |
| CD | Crohn’s disease |
| SCFAs | Short-chain fatty acids |
| NF-κB p65 | Nuclear factor kappa-B p65 |
| NF-κB | Nuclear factor kappa-B |
| LRR | Leucine-rich repeat |
| PYD | Pyrin domain |
| LPS | Lipopolysaccharide |
| IL-1β | Interleukin -1β |
| PGE-2 | Prostaglandin E2 |
| CMC | Sodium carboxymethyl cellulose |
| ELISA | Enzyme-linked immunosorbent assay |
| SPF | Specific pathogen-free |
| CON | Control |
| HRP | Horseradish peroxidase |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
| H&E | Colonic hematoxylin and eosin |
| QC | Quality control |
| AGC | Automatic gain control |
| DNA | Deoxyribonucleic acid |
| PCR | Polymerase chain reaction |
| QIIME2 | Quantitative Insights Into Microbial Ecology version 2 |
| PCoA | Principal coordinate analysis |
| NMDS | Non-metric multidimensional scaling analysis |
| DADA2 | Divisive Amplicon Denoising Algorithm |
| LC-MS | Liquid chromatograph mass spectrometer |
| PCA | Principal component analysis |
| LEfSe | Linear discriminant analysis effective size |
| PLS-DA | Partial least squares discriminant analysis |
| VIP | Variable Importance in Projection |
| DA score | Differential abundance score |
| OTUs | Operational Taxonomic Units |
| iNOS | Inducible nitric oxidesynthase |
| ALS | Amyotrophic lateral sclerosis |
| NAFLD | Non-alcoholic fatty liver disease |
| TGR5 | Takeda G protein-coupled receptor 5 |
References
- Andlujar, I.; Recio, M.C.; Giner, R.M.; Cienfuegos-Jovellanos, E.; Laghi, S.; Muguerza, B.; Rios, J.L. Inhibition of Ulcerative Colitis in Mice after Oral Administration of a Polyphenol-Enriched Cocoa Extract Is Mediated by the Inhibition of STAT1 and STAT3 Phosphorylation in Colon Cells. J. Agric. Food Chem. 2011, 59, 6474–6483. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, G.; Malekshahi, H.; Miraghaee, S.; Madani, H.; Babaei, A. Improving Animal Model of Induced Colitis by Acetic Acid in Terms of Fibrosis and Inflammation Incidence in the Colon. J. Investig. Surg. 2022, 35, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Julieta Gonzalez, M.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277, Erratum in Front. Immunol. 2019, 10, 1486. [Google Scholar] [CrossRef] [PubMed]
- Low, D.; Nguyen, D.D.; Mizoguchi, E. Animal models of ulcerative colitis and their application in drug research. Drug Des. Dev. Ther. 2013, 7, 1341–1356. [Google Scholar] [CrossRef]
- Isaacs, K.L.; Lewis, J.D.; Sandborn, W.J.; Sands, B.E.; Targan, S.R. State of the art: IBD therapy and clinical trials in IBD. Inflamm. Bowel Dis. 2005, 11, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Irving, P.M. Optimization of conventional therapy in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 646–656. [Google Scholar] [CrossRef]
- Nidhi; Rashid, M.; Kaur, V.; Hallan, S.S.; Sharma, S.; Mishra, N. Microparticles as controlled drug delivery carrier for the treatment of ulcerative colitis: A brief review. Saudi Pharm. J. 2016, 24, 458–472. [Google Scholar] [CrossRef]
- Cao, S.-Y.; Ye, S.-J.; Wang, W.-W.; Wang, B.; Zhang, T.; Pu, Y.-Q. Progress in active compounds effective on ulcerative colitis from Chinese medicines. Chin. J. Nat. Med. 2019, 17, 81–102. [Google Scholar] [CrossRef]
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The Anti-inflammatory Effects of Dietary Anthocyanins against Ulcerative Colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Albenberg, L.; Compher, C.; Baldassano, R.; Piccoli, D.; Lewis, J.D.; Wu, G.D. Diet in the Pathogenesis and Treatment of Inflammatory Bowel Diseases. Gastroenterology 2015, 148, 1087–1106. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.F.Z.; Kim, D.V.; Norwood, K.; Saldana-Morales, F.B.; Kim, M.; Ng, C.; Callaghan, R.; Uddin, M.; Chang, L.C.; Longman, R.S.; et al. Microbiota manipulation to increase macrophage IL-10 improves colitis and limits colitis-associated colorectal cancer. Gut Microbes 2022, 14, 2119054. [Google Scholar] [CrossRef]
- Askari, H.; Shojaei-Zarghani, S.; Raeis-Abdollahi, E.; Jahromi, H.K.; Abdullahi, P.R.; Daliri, K.; Tajbakhsh, A.; Rahmati, L.; Safarpour, A.R. The Role of Gut Microbiota in Inflammatory Bowel Disease-Current State of the Art. Mini-Rev. Med. Chem. 2023, 23, 1376–1389. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Unno, T.; Kim, B.-Y.; Park, M.-S. Sex Differences in Gut Microbiota. World J. Mens Health 2020, 38, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Park, Y.-H.; Sim, M.; Kim, S.-A.; Joung, H.; Shin, D.-M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res. Microbiol. 2019, 170, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lovell, R.M.; Ford, A.C. Effect of Gender on Prevalence of Irritable Bowel Syndrome in the Community: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2012, 107, 991–1000. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, N.; Li, Z.; Fu, D.; Guo, Y.; Gao, X.; Liu, X. Co-occurrence of gut microbiota dysbiosis and bile acid metabolism alteration is associated with psychological disorders in Crohn’s disease. Faseb J. 2022, 36, e22100. [Google Scholar] [CrossRef]
- Shadnoush, M.; Hosseini, R.S.; Navai, L.; Goudarzi, H.; Vaezjalali, M. Effects of Probiotics on Gut Microbiota in Patients with Inflammatory Bowel Disease: A Double-blind, Placebo-controlled Clinical Trial. Korean J. Gastroenterol. 2015, 65, 215–221. [Google Scholar] [CrossRef]
- Leccese, G.; Bibi, A.; Mazza, S.; Facciotti, F.; Caprioli, F.; Landini, P.; Paroni, M. Probiotic Lactobacillus and Bifidobacterium Strains Counteract Adherent-Invasive Escherichia coli (AIEC) Virulence and Hamper IL-23/Th17 Axis in Ulcerative Colitis, but Not in Crohn’s Disease. Cells 2020, 9, 1824. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef] [PubMed]
- Collino, S.; Martin, F.P.J.; Rezzi, S. Clinical metabolomics paves the way towards future healthcare strategies. Br. J. Clin. Pharmacol. 2013, 75, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.-N.; Wang, M.; Guo, J.; Wang, J.-P. Role of intestinal microbiota and metabolites in inflammatory bowel disease. Chin. Med. J. 2019, 132, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Haesler, R.; et al. Increased Tryptophan Metabolism Is Associated With Activity of Inflammatory Bowel Diseases. Gastroenterology 2017, 153, 1504–1516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Commun. Signal. 2022, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Inca, R.; et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef] [PubMed]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [CrossRef]
- Goncalves, P.; Araujo, J.R.; Di Santo, J.P. A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid levels in commercial basil (Ocimum basilicum) and Echinacea purpurea products. J. Funct. Foods 2010, 2, 77–84. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid: Chemistry, distribution, and production. Front. Chem. 2013, 1, 40. [Google Scholar] [CrossRef]
- Lee, N.Y.; Chung, K.-S.; Jin, J.S.; Bang, K.S.; Eom, Y.-J.; Hong, C.-H.; Nugroho, A.; Park, H.-J.; An, H.-J. Effect of Chicoric Acid on Mast Cell-Mediated Allergic Inflammation in Vitro and in Vivo. J. Nat. Prod. 2015, 78, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, S.; Du, M.; Zhu, M.-J. Dandelion extract suppresses reactive oxidative species and inflammasome in intestinal epithelial cells. J. Funct. Foods 2017, 29, 10–18. [Google Scholar] [CrossRef]
- Jiang, L.; Li, W.; Wang, Y.; Zhang, X.; Yu, D.; Yin, Y.; Xie, Z.; Yuan, Y. Effects of Cichoric Acid Extract from Echinacea purpurea on Collagen-Induced Arthritis in Rats. American J. Chin. Med. 2014, 42, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Srinivasan, G.; Delgado, M.A.; Young, A.N.; Gewirtz, A.T.; Vijay-Kumar, M. Fecal Lipocalin 2, a Sensitive and Broadly Dynamic Non-Invasive Biomarker for Intestinal Inflammation. PLoS ONE 2012, 7, e44328. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, M.; Kristensen, M.N.; Venkatasubramanian, R.; Guerra, P.; Jacobsen, J.; Berthelsen, R.; Rades, T.; Mullertz, A. Assessing acute colitis induced by dextran sulfate sodium in rats and its impact on gastrointestinal fluids. Drug Deliv. Transl. Res. 2023, 13, 1484–1499. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.W.; Niu, H.T. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.; Shen, C.; Xiao, Y.; Wang, Y.; Liu, Z.; Liu, X. Chicoric acid supplementation prevents systemic inflammation- induced memory impairment and amyloidogenesis via inhibition of NF-kappa B. Faseb J. 2017, 31, 1494–1507. [Google Scholar] [CrossRef]
- Landmann, M.; Kanuri, G.; Spruss, A.; Stahl, C.; Bergheim, I. Oral intake of chicoric acid reduces acute alcohol-induced hepatic steatosis in mice. Nutrition 2014, 30, 882–889. [Google Scholar] [CrossRef]
- Michielan, A.; D’Inca, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediat. Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef]
- Gancarcikova, S.; Lauko, S.; Hrckova, G.; Andrejcakova, Z.; Hajduckova, V.; Madar, M.; Fecskeova, L.K.; Mudronova, D.; Mravcova, K.; Strkolcova, G.; et al. Innovative Animal Model of DSS-Induced Ulcerative Colitis in Pseudo Germ-Free Mice. Cells 2020, 9, 2571. [Google Scholar] [CrossRef]
- Sheehan, D.; Moran, C.; Shanahan, F. The microbiota in inflammatory bowel disease. J. Gastroenterol. 2015, 50, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Walujkar, S.A.; Dhotre, D.P.; Marathe, N.P.; Lawate, P.S.; Bharadwaj, R.S.; Shouche, Y.S. Characterization of bacterial community shift in human Ulcerative Colitis patients revealed by Illumina based 16S rRNA gene amplicon sequencing. Gut Pathog. 2014, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, B.-N.; Hu, B.; Cheng, Y.-L.; Guo, Y.-H.; Qian, H. Neuroprotection of chicoric acid in a mouse model of Parkinson’s disease involves gut microbiota and TLR4 signaling pathway. Food Funct. 2022, 13, 2019–2032. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.Q.; Jian, T.Y.; Li, J.W.; Lv, H.; Tong, B.; Li, J.; Meng, X.H.; Ren, B.R.; Chen, J. Chicoric Acid Ameliorates Nonalcoholic Fatty Liver Disease via the AMPK/Nrf2/NF kappa B Signaling Pathway and Restores Gut Microbiota in High-Fat-Diet-Fed Mice. Oxidative Med. Cell. Longev. 2020, 2020, 9734560. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, D.; Bozzi Cionci, N.; Baffoni, L.; Amoruso, A.; Pane, M.; Mogna, L.; Gaggia, F.; Lucenti, M.A.; Bersano, E.; Cantello, R.; et al. A prospective longitudinal study on the microbiota composition in amyotrophic lateral sclerosis. Bmc Med. 2020, 18, 153. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Zheng, J.; Hu, G.; Wang, J.; Huang, C.; Lou, L.; Wang, X.; Zeng, Y. Mucosal adherent bacterial dysbiosis in patients with colorectal adenomas. Sci. Rep. 2016, 6, 26337. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Li, Y.; Li, J.; Yang, J.; Shang, L.; He, X.; Liu, L.; Luo, Y.; Xie, X. Intestinal microbiota profiles in infants with acute gastroenteritis caused by rotavirus and norovirus infection: A prospective cohort study. Int. J. Infect. Dis. 2021, 111, 76–84. [Google Scholar] [CrossRef]
- Chumpitazi, B.P.; Hoffman, K.L.; Smith, D.P.; McMeans, A.R.; Musaad, S.; Versalovic, J.; Petrosino, J.F.; Shulman, R.J. Fructan-sensitive children with irritable bowel syndrome have distinct gut microbiome signatures. Aliment. Pharmacol. Ther. 2021, 53, 499–509. [Google Scholar] [CrossRef]
- Huang, C.; Chen, J.; Wang, J.; Zhou, H.; Lu, Y.; Lou, L.; Zheng, J.; Tian, L.; Wang, X.; Cao, Z.; et al. Dysbiosis of Intestinal Microbiota and Decreased Antimicrobial Peptide Level in Paneth Cells during Hypertriglyceridemia-Related Acute Necrotizing Pancreatitis in Rats. Front. Microbiol. 2017, 8, 776. [Google Scholar] [CrossRef]
- Tang, R.; Jiang, Y.; Tan, A.; Ye, J.; Xian, X.; Xie, Y.; Wang, Q.; Yao, Z.; Mo, Z. 16S rRNA gene sequencing reveals altered composition of gut microbiota in individuals with kidney stones. Urolithiasis 2018, 46, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Q.-F.; Zheng, J.; Yuan, B.-F.; Feng, Y.-Q. Mass spectrometry-based fecal metabolome analysis. Trac-Trends Anal. Chem. 2019, 112, 161–174. [Google Scholar] [CrossRef]
- Owczarek, D.; Cibor, D.; Mach, T. Asymmetric Dimethylarginine (ADMA), Symmetric Dimethylarginine (SDMA), Arginine, and 8-Iso-Prostaglandin F2 alpha (8-iso-PGF2 alpha) Level in Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2010, 16, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh-Attar, M.J.; Sharifi, A.; Nedjat, S.; Mohamadkhani, A.; Vahedi, H. The Effect of Vitamin D on Serum Asymmetric Dimethylarginine in Patients with Mild to Moderate Ulcerative Colitis. Int. J. Vitam. Nutr. Res. 2020, 90, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Y.; Zhao, A.; Qiu, Y.; Xie, G.; Jiang, Q.; Zheng, X.; Zhong, W.; Sun, X.; Zhou, Z.; et al. Asymmetric dimethylarginine attenuates serum starvation-induced apoptosis via suppression of the Fas (APO-1/CD95)/JNK (SAPK) pathway. Cell Death Dis. 2013, 4, e830. [Google Scholar] [CrossRef]
- Samraj, A.N.; Bertrand, K.A.; Luben, R.; Khedri, Z.; Yu, H.; Nguyen, D.; Gregg, C.J.; Diaz, S.L.; Sawyer, S.; Chen, X.; et al. Polyclonal human antibodies against glycans bearing red meat-derived non-human sialic acid N-glycolylneuraminic acid are stable, reproducible, complex and vary between individuals: Total antibody levels are associated with colorectal cancer risk. PLoS ONE 2018, 13, e0197464. [Google Scholar] [CrossRef]
- Jahan, M.; Thomson, P.C.; Wynn, P.C.; Wang, B. Red Meat Derived Glycan, N-acetylneuraminic Acid (Neu5Ac) Is a Major Sialic Acid in Different Skeletal Muscles and Organs of Nine Animal Species-A Guideline for Human Consumers. Foods 2023, 12, 337. [Google Scholar] [CrossRef]
- Kim, M.-M.; Kim, S.-K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010, 48, 2925–2933. [Google Scholar] [CrossRef]
- Owczarek, D.; Rodacki, T.; Domagala-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef]
- Vitali, R.; Prioreschi, C.; Rebenaque, L.L.; Colantoni, E.; Giovannini, D.; Frusciante, S.; Diretto, G.; Marco-Jimenez, F.; Mancuso, M.; Casciati, A.; et al. Gut-Brain Axis: Insights from Hippocampal Neurogenesis and Brain Tumor Development in a Mouse Model of Experimental Colitis Induced by Dextran Sodium Sulfate. Int. J. Mol. Sci. 2022, 23, 11495. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Lam, C.W.; Curreem, S.O.T.; Lee, K.C.; Chow, W.N.; Lau, C.C.Y.; Sridhar, S.; Wong, S.C.Y.; Martelli, P.; Hui, S.W.; et al. Metabolomic profiling of Burkholderia pseudomallei using UHPLC-ESI-Q-TOF-MS reveals specific biomarkers including 4-methyl-5-thiazoleethanol and unique thiamine degradation pathway. Cell Biosci. 2015, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.-U.; Backhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 2020, 27, 659–670.e5. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.; Schoonjans, K. MOLECULAR PHYSIOLOGY OF BILE ACID SIGNALING IN HEALTH, DISEASE, AND AGING. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Sleiman, M.B.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology 2020, 159, 956–968.e8. [Google Scholar] [CrossRef]
- Liang, J.; Chang, B.; Huang, M.; Huang, W.; Ma, W.; Liu, Y.; Tai, W.; Long, Y.; Lu, Y. Oxymatrine prevents synovial inflammation and migration via blocking NF-kappa B activation in rheumatoid fibroblast-like synoviocytes. Int. Immunopharmacol. 2018, 55, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.R.; Koo, J.S.; Goldsmith, J.R.; Muehlbauer, M.; Narula, A.; Jobin, C. Oxymatrine Prevents NF-kappa B Nuclear Translocation And Ameliorates Acute Intestinal Inflammation. Sci. Rep. 2013, 3, 1629. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liu, W.; Zhao, Q.; Li, K.; Zhu, J.; Yao, W.; Gao, X. Combination of polysaccharides from Astragalus membranaceus and Codonopsis pilosula ameliorated mice colitis and underlying mechanisms. J. Ethnopharmacol. 2021, 264, 113280. [Google Scholar] [CrossRef]
- Backer, V.; Cheung, F.Y.; Siveke, J.T.; Fandrey, J.; Winning, S. Knockdown of myeloid cell hypoxia-inducible factor-1 alpha ameliorates the acute pathology in DSS-induced colitis. PLoS ONE 2017, 12, e0190074. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Zhou, J.; Hong, H.; Zhao, J.; Fang, R.; Chen, S.; Tang, C. Metabolome analysis to investigate the effect of heavy metal exposure and chemoprevention agents on toxic injury caused by a multi-heavy metal mixture in rats. Sci. Total Environ. 2024, 906, 167513. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Tan, H.; Shi, Y.; Xu, M.; Luo, S.; Weng, J.; Xu, S. Serum Metabolomic Profiling in Aging Mice Using Liquid Chromatography-Mass Spectrometry. Biomolecules 2022, 12, 1594. [Google Scholar] [CrossRef] [PubMed]





| Metabolite | Fold Change | p-Value | VIP | DSS vs. CON | CA vs. DSS | CA vs. CON |
|---|---|---|---|---|---|---|
| Asymmetric dimethylarginine | 0.6509 | 0.0403 | 1.2106 | ↑ | ↓ | ↑ |
| D-(+)-Raffinose | 0.4744 | 0.0071 | 1.5651 | ↑ | ↓ | ↑ |
| N-Acetyl-D-glucosamine | 0.4189 | 0.0348 | 1.5544 | ↑ | ↓ | n.s. |
| Quinone sulfate | 0.1482 | 0.0248 | 3.6340 | ↑ | ↓ | n.s. |
| Xanthurenic acid | 0.245 | 0.0126 | 2.1214 | ↑ | ↓ | n.s. |
| 4-hydroxy-2-quinolinecarboxylic acid | 1.8232 | 0.0039 | 1.5071 | ↓ | ↑ | n.s. |
| 1H-Indole-2,3-dione | 0.5489 | 0.0261 | 1.4475 | ↑ | ↓ | n.s. |
| Gamma-linolenic acid | 0.6312 | 0.0408 | 1.1922 | ↑ | ↓ | n.s. |
| Thiamine | 2.0784 | 0.0054 | 1.4288 | ↓ | ↑ | n.s. |
| 4-Methyl-5-thiazoleethanol | 2.1456 | 0.0046 | 1.5127 | ↓ | ↑ | n.s. |
| N-Glycolylneuraminic acid | 0.0199 | 0.0319 | 2.9523 | ↑ | ↓ | n.s. |
| Allantoin | 0.0886 | 0.0491 | 2.8324 | ↑ | ↓ | n.s. |
| N-Acetylneuraminic acid | 0.1702 | 0.0356 | 2.0773 | ↑ | ↓ | n.s. |
| Phloroglucinol | 3.5918 | 0.0181 | 2.0928 | ↓ | ↑ | n.s. |
| Alpha -Aspartylphenylalanine | 0.2381 | 0.0487 | 1.1902 | ↑ | ↓ | n.s. |
| Lithocholic acid | 1.9365 | 0.0176 | 1.4303 | ↓ | ↑ | n.s. |
| Lipoic acid | 0.5566 | 0.0216 | 1.2972 | ↑ | ↓ | n.s. |
| D-(−)-Quinic acid | 0.3136 | 0.0196 | 1.8506 | ↑ | ↓ | n.s. |
| L-Alanine | 0.3494 | 0.0040 | 2.0185 | ↑ | ↓ | n.s. |
| Piperine | 1.3955 | 0.0440 | 1.2827 | ↓ | ↑ | n.s. |
| Oxymatrine | 2.4038 | 0.0063 | 1.2889 | ↓ | ↑ | n.s. |
| 2-Hydroxy-4-(hydroxymethyl)-6-(1-hydroxy-3-methylbut-2-enyl)-3-[(E)-prop-1-enyl]-7-oxabicyclo[4.1.0]hept-3-en-5-one | 1.8753 | 0.0037 | 1.4079 | ↓ | ↑ | n.s. |
| Ibuprofen | 0.3316 | 0.0118 | 1.4320 | ↑ | ↓ | n.s. |
| 15-Deoxy-delta 12,14-prostaglandins D2 | 2.74 | 0.0003 | 2.0166 | ↓ | ↑ | n.s. |
| Thymidine 5′-monophosphate | 0.1015 | 0.0162 | 2.6353 | ↑ | ↓ | n.s. |
| Arachidonoyl ethanolamide phosphate | 0.5875 | 0.0201 | 1.5616 | ↑ | ↓ | n.s. |
| (S)-AL 8810 | 0.4339 | 0.0208 | 2.1068 | ↑ | ↓ | n.s. |
| 14-(Hydroxymethyl)-5,9-dimethyltetracyclo[11.2.1.0] | 1.535 | 0.0295 | 1.2260 | ↓ | ↑ | n.s. |
| 11-Deoxy prostaglandin F2 | 0.4026 | 0.0184 | 1.2333 | ↑ | ↓ | n.s. |
| Skatole | 0.7237 | 0.0074 | 1.1165 | n.s. | ↓ | n.s. |
| Alpha-tocopherol acetate | 16.971 | 0.0184 | 2.6740 | n.s. | ↑ | n.s. |
| Ursolic acid | 1.5638 | 0.0385 | 1.1528 | n.s. | ↑ | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Lin, J.; Gu, T.; Sun, Q.; Xu, W.; Peng, Y. Chicoric Acid Effectively Mitigated Dextran Sulfate Sodium (DSS)-Induced Colitis in BALB/c Mice by Modulating the Gut Microbiota and Fecal Metabolites. Int. J. Mol. Sci. 2024, 25, 841. https://doi.org/10.3390/ijms25020841
Yang J, Lin J, Gu T, Sun Q, Xu W, Peng Y. Chicoric Acid Effectively Mitigated Dextran Sulfate Sodium (DSS)-Induced Colitis in BALB/c Mice by Modulating the Gut Microbiota and Fecal Metabolites. International Journal of Molecular Sciences. 2024; 25(2):841. https://doi.org/10.3390/ijms25020841
Chicago/Turabian StyleYang, Jiani, Jie Lin, Ting Gu, Quancai Sun, Weidong Xu, and Ye Peng. 2024. "Chicoric Acid Effectively Mitigated Dextran Sulfate Sodium (DSS)-Induced Colitis in BALB/c Mice by Modulating the Gut Microbiota and Fecal Metabolites" International Journal of Molecular Sciences 25, no. 2: 841. https://doi.org/10.3390/ijms25020841