Ex Vivo-Generated Tolerogenic Dendritic Cells: Hope for a Definitive Therapy of Autoimmune Diseases
Abstract
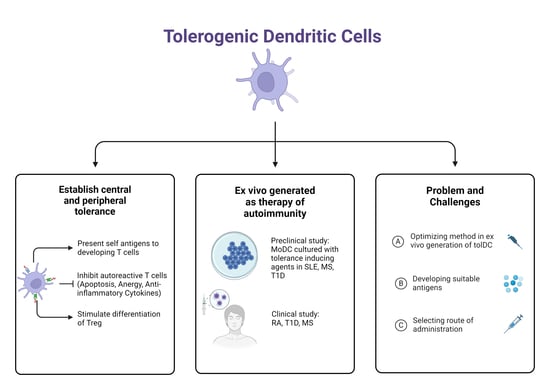
:1. Introduction
2. The Role of Dendritic Cells in Autoimmunity
2.1. Central Tolerance
2.2. Peripheral Tolerance
2.3. Role of Dendritic Cells in SLE
3. Recent Developments in Ex Vivo-Generated Tolerogenic Dendritic Cell Therapy for Autoimmune Diseases
4. Therapeutic Potential, Problem, and Challenges of Dendritic Cell Immunotherapy for Autoimmune Diseases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Ameer, M.A.; Chaudhry, H.; Mushtaq, J.; Khan, O.S.; Babar, M.; Hashim, T.; Zeb, S.; Tariq, M.A.; Patlolla, S.R.; Ali, J.; et al. An Overview of Systemic Lupus Erythematosus (SLE) Pathogenesis, Classification, and Management. Cureus 2022, 14, e30330. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Morand, E.F.; Fernandez-Ruiz, R.; Blazer, A.; Niewold, T.B. Advances in the management of systemic lupus erythematosus. BMJ 2023, 383, e073980. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Chalhoub, N.E.; Sherwin, C.M.; Li, C.; Brunner, H.I. Glucocorticoids pharmacology and their application in the treatment of childhood-onset systemic lupus erythematosus. Semin. Arthritis Rheum. 2019, 49, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.-Y.; Chen, H.-T.; Liang, S.-Q.; Yang, C.; Yao, C.-W.; Xu, Y.-Z.; Liu, H.-F.; An, N. Revisited Cyclophosphamide in the Treatment of Lupus Nephritis. BioMed. Res. Int. 2022, 2022, 8345737. [Google Scholar] [CrossRef] [PubMed]
- Mosanya, C.H.; Isaacs, J.D. Tolerising cellular therapies: What is their promise for autoimmune disease? Ann. Rheum. Dis. 2018, 78, 297–310. [Google Scholar] [CrossRef]
- Hilkens, C.M.U.; Isaacs, J.D. Tolerogenic dendritic cell therapy for rheumatoid arthritis: Where are we now? Clin. Exp. Immunol. 2013, 172, 148–157. [Google Scholar] [CrossRef]
- Passeri, L.; Marta, F.; Bassi, V.; Gregori, S. Tolerogenic Dendritic Cell-Based Approaches in Autoimmunity. Int. J. Mol. Sci. 2021, 22, 8415. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Systemic Lupus Erythematosus and DNA Degradation and Elimination Defects. Front. Immunol. 2019, 10, 1697. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Podolska, M.; Biermann, M.; Maueröder, C.; Hahn, J. Inflammatory etiopathogenesis of systemic lupus erythematosus: An update. J. Inflamm. Res. 2015, 8, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Ahlers, M.J.; Lowery, B.D.; Farber-Eger, E.; Wang, T.J.; Bradham, W.; Ormseth, M.J.; Chung, C.P.; Stein, C.M.; Gupta, D.K. Heart Failure Risk Associated With Rheumatoid Arthritis–Related Chronic Inflammation. J. Am. Hear. Assoc. 2020, 9, e014661. [Google Scholar] [CrossRef] [PubMed]
- AlFaris, H.; Alkathiri, S.A.; Babelli, D.; Alokaily, F. Double malignancy and a mycobacterial infection in a rheumatoid arthritis patient. SciVee 2022, 43, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.K.; Reynolds, K.M.; Poole, B.D.; Montierth, M.D.; Todd, V.M.; Barnado, A.; Davis, M.F. Contribution of viral infection to risk for cancer in systemic lupus erythematosus and multiple sclerosis. PLoS ONE 2021, 16, e0243150. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Latorre, G.; Ivanovic-Zuvic, D.; Camargo, M.C.; Rabkin, C.S. Autoimmune diseases and gastric cancer risk: A systematic review and meta-analysis. Cancer Res. Treat. 2019, 51, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Waisman, A.; Lukas, D.; Clausen, B.E.; Yogev, N. Dendritic cells as gatekeepers of tolerance. Semin. Immunopathol. 2016, 39, 153–163. [Google Scholar] [CrossRef]
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic Cell Subsets and Locations, 1st ed.; International Review of Cell and Molecular Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 348, pp. 1–68. [Google Scholar]
- Saadeh, D.; Kurban, M.; Abbas, O. Update on the role of plasmacytoid dendritic cells in inflammatory/autoimmune skin diseases. Exp. Dermatol. 2016, 25, 415–421. [Google Scholar] [CrossRef]
- Takagi, H.; Arimura, K.; Uto, T.; Fukaya, T.; Nakamura, T.; Choijookhuu, N.; Hishikawa, Y.; Sato, K. Plasmacytoid dendritic cells orchestrate TLR7-mediated innate and adaptive immunity for the initiation of autoimmune inflammation. Sci. Rep. 2016, 6, 24477. [Google Scholar] [CrossRef]
- Kisielow, P. How does the immune system learn to distinguish between good and evil? The first definitive studies of T cell central tolerance and positive selection. Immunogenetics 2019, 71, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Macri, C.; Pang, E.S.; Patton, T.; O’Keeffe, M. Dendritic cell subsets. Semin. Cell Dev. Biol. 2018, 84, 11–21. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, J. Properties of immature and mature dendritic cells: Phenotype, morphology, phagocytosis, and migration. RSC Adv. 2019, 9, 11230–11238. [Google Scholar] [CrossRef] [PubMed]
- Ugur, M.; Mueller, S.N. T cell and dendritic cell interactions in lymphoid organs: More than just being in the right place at the right time. Immunol. Rev. 2019, 289, 115–128. [Google Scholar] [CrossRef]
- Hilligan, K.L.; Ronchese, F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell. Mol. Immunol. 2020, 17, 587–599. [Google Scholar] [CrossRef]
- Roche, P.A.; Cresswell, P. Antigen processing and presentation mechanisms in myeloid cells. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Klein, L.; Hinterberger, M.; Wirnsberger, G.; Kyewski, B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat. Rev. Immunol. 2009, 9, 833–844. [Google Scholar] [CrossRef]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology, 9th ed.; Garland Science: New York, NY, USA; Taylor & Francis Group: London, UK, 2017. [Google Scholar]
- Czaja, A.J.; Santrach, P.J.; Moore, S.B. Shared Genetic Risk Factors in Autoimmune Liver Disease. Dig. Dis. Sci. 2001, 46, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.R.; Kragstrup, T.W.; Deleuran, B.W.; Benros, M.E. Infections as risk factor for autoimmune diseases—A nationwide study. J. Autoimmun. 2016, 74, 176–181. [Google Scholar] [CrossRef]
- Proietto, A.I.; van Dommelen, S.; Zhou, P.; Rizzitelli, A.; D’Amico, A.; Steptoe, R.J.; Naik, S.H.; Lahoud, M.H.; Liu, Y.; Zheng, P.; et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc. Natl. Acad. Sci. USA 2008, 105, 19869–19874. [Google Scholar] [CrossRef] [PubMed]
- Metzger, T.C.; Anderson, M.S. Control of central and peripheral tolerance by Aire. Immunol. Rev. 2011, 241, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, Ø.; Oftedal, B.E.; Wolff, A.B.; Husebye, E.S. AIRE-mutations and autoimmune disease. Curr. Opin. Immunol. 2016, 43, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Borchers, J.; Pukkala, E.; Mäkitie, O.; Laakso, S. Patients with APECED Have Increased Early Mortality Due to Endocrine Causes, Malignancies and infections. J. Clin. Endocrinol. Metab. 2020, 105, e2207-13. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Wei, J.; Zhang, A.; Gong, W.; Fu, J.; Jia, T.; Yang, S.-Y. Antigen-specific tolerogenic dendritic cells ameliorate the severity of murine collagen-induced arthritis. PLoS ONE 2015, 10, e0131152. [Google Scholar] [CrossRef] [PubMed]
- Funes, S.C.; de Lara, A.M.; Altamirano-Lagos, M.J.; Mackern-Oberti, J.P.; Escobar-Vera, J.; Kalergis, A.M. Immune checkpoints and the regulation of tolerogenicity in dendritic cells: Implications for autoimmunity and immunotherapy. Autoimmun. Rev. 2019, 18, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Malinarich, F.; Duan, K.; Hamid, R.A.; Bijin, A.; Lin, W.X.; Poidinger, M.; Fairhurst, A.-M.; Connolly, J.E. High Mitochondrial Respiration and Glycolytic Capacity Represent a Metabolic Phenotype of Human Tolerogenic Dendritic Cells. J. Immunol. 2015, 194, 5174–5186. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-Y.; Lucavs, J.; Ballard, D.; Das, J.K.; Kumar, A.; Wang, L.; Ren, Y.; Xiong, X.; Song, J. Metabolic Reprogramming and Reactive Oxygen Species in T Cell Immunity. Front. Immunol. 2021, 12, 652687. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.O.; van der Kooij, S.W.; Vignali, D.A.A.; van Kooten, C. Human tolerogenic dendritic cells produce IL-35 in the absence of other IL-12 family members. Eur. J. Immunol. 2015, 45, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Lu, M.-P.; Wang, J.-H.; Xu, M.; Yang, S.-R. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J. Pediatr. 2019, 16, 19–30. [Google Scholar] [CrossRef]
- Kaewraemruaen, C.; Ritprajak, P.; Hirankarn, N. Dendritic cells as key players in systemic lupus erythematosus. Asian Pac. J. Allergy Immunol. 2020, 38, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.-H.; Cohen, P.L. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res. Ther. 2010, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S.; Lipsky, P.E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2020, 16, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-M.; Tsokos, G.C. T Cell Abnormalities in the Pathogenesis of Systemic Lupus Erythematosus: An Update. Curr. Rheumatol. Rep. 2021, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Lo, Y.; Luk, D.; Lau, C.S.; Lu, L.; Mok, M.Y. Alternatively activated dendritic cells derived from systemic lupus erythematosus patients have tolerogenic phenotype and function. Clin. Immunol. 2015, 156, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Obreque, J.; Vega, F.; Torres, A.; Cuitino, L.; Mackern-Oberti, J.P.; Viviani, P.; Kalergis, A.; Llanos, C. Autologous tolerogenic dendritic cells derived from monocytes of systemic lupus erythematosus patients and healthy donors show a stable and immunosuppressive phenotype. Immunology 2017, 152, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Capetillo, L.; Hernández-Castro, B.; Monsiváis-Urenda, A.; Alvarez-Quiroga, C.; Layseca-Espinosa, E.; Abud-Mendoza, C.; Baranda, L.; Urzainqui, A.; Sánchez-Madrid, F.; González-Amaro, R. Induction of Th17 Lymphocytes and Treg Cells by Monocyte-Derived Dendritic Cells in Patients with Rheumatoid Arthritis and Systemic Lupus Erythematosus. J. Immunol. Res. 2013, 2013, 584303. [Google Scholar] [CrossRef]
- Esmaeili, S.; Mahmoudi, M.; Rezaieyazdi, Z.; Sahebari, M.; Tabasi, N.; Sahebkar, A.; Rastin, M. Generation of tolerogenic dendritic cells using Lactobacillus rhamnosus and Lactobacillus delbrueckii as tolerogenic probiotics. J. Cell. Biochem. 2018, 119, 7865–7872. [Google Scholar] [CrossRef] [PubMed]
- Nikpoor, A.R.; Mahmoudi, M.; Shapouri-Moghaddam, A.; Rezaieyazdi, Z.; Mollazadeh, S.; Tabasi, N.; Mansouri, A.; Moghadam, R.M.; Momtazi, A.A.; Najmaldin, S.K.; et al. Curcumin and Berberine Arrest Maturation and Activation of Dendritic Cells Derived from Lupus Erythematosus Patients. Curr. Mol. Pharmacol. 2024, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, J.; Zheng, C.; Wu, J.; Cheng, Y.; Zhu, S.; Lin, C.; Cao, Q.; Zhu, J.; Jin, T. 1,25-dihydroxyvitamin D3-induced dendritic cells suppress experimental autoimmune encephalomyelitis by increasing proportions of the regulatory lymphocytes and reducing T helper type 1 and type 17 cells. Immunology 2017, 152, 414–424. [Google Scholar] [CrossRef]
- Li, C.H.; Zhang, J.; Baylink, D.J.; Wang, X.; Goparaju, N.B.; Xu, Y.; Wasnik, S.; Cheng, Y.; Berumen, E.C.; Qin, X.; et al. Dendritic cells, engineered to overexpress 25-hydroxyvitamin D 1α-hydroxylase and pulsed with a myelin antigen, provide myelin-specific suppression of ongoing experimental allergic encephalomyelitis. FASEB J. 2017, 31, 2996–3006. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Xia, C.-Q.; Peng, R.; Clare-Salzler, M.J. Immature Dendritic Cell Therapy Confers Durable Immune Modulation in an Antigen-Dependent and Antigen-Independent Manner in Nonobese Diabetic Mice. J. Immunol. Res. 2018, 2018, 5463879. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.M.; Anderson, A.E.; Diboll, J.; Reece, R.; Eltherington, O.; Harry, R.A.; Fouweather, T.; MacDonald, C.; Chadwick, T.; McColl, E.; et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann. Rheum. Dis. 2016, 76, 227–234. [Google Scholar] [CrossRef]
- Giannoukakis, N.; Phillips, B.; Finegold, D.; Harnaha, J.; Trucco, M. Phase I (Safety) Study of Autologous Tolerogenic Dendritic Cells in Type 1 Diabetic Patients. Diabetes Care 2011, 34, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Zubizarreta, I.; Flórez-Grau, G.; Vila, G.; Cabezón, R.; España, C.; Andorra, M.; Saiz, A.; Llufriu, S.; Sepulveda, M.; Sola-Valls, N.; et al. Immune tolerance in multiple sclerosis and neuromyelitis optica with peptide-loaded tolerogenic dendritic cells in a phase 1b trial. Proc. Natl. Acad. Sci. USA 2019, 116, 8463–8470. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, T.; Suwandi, J.S.; Wesselius, J.; Laban, S.; Joosten, A.M.; Sonneveld, P.; Mul, D.; Aanstoot, H.-J.; Kaddis, J.S.; Zwaginga, J.J.; et al. Tolerogenic dendritic cells pulsed with islet antigen induce long-term reduction in T-cell autoreactivity in type 1 diabetes patients. Front. Immunol. 2022, 13, 1054968. [Google Scholar] [CrossRef] [PubMed]
- AHorwitz, D. Regulatory T cells in systemic lupus erythematosus: Past, present and future. Arthritis Res. Ther. 2008, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.; Chintala, S.; Dey, M. Plasmacytoid dendritic cell in immunity and cancer. J. Neuroimmunol. 2018, 322, 63–73. [Google Scholar] [CrossRef]
- Tiberio, L.; Del Prete, A.; Schioppa, T.; Sozio, F.; Bosisio, D.; Sozzani, S. Chemokine and chemotactic signals in dendritic cell migration review-article. Cell Mol. Immunol. 2018, 15, 346–352. [Google Scholar] [CrossRef]
- Lorenz, G.; Anders, H.J. Neutrophils, Dendritic Cells, Toll-Like Receptors, and Interferon-α in Lupus Nephritis. Semin. Nephrol. 2015, 35, 410–426. [Google Scholar] [CrossRef]
- Funes, S.C.; Ríos, M.; Gómez-Santander, F.; Fernández-Fierro, A.; Altamirano-Lagos, M.J.; Rivera-Perez, D.; Pulgar-Sepúlveda, R.; Jara, E.L.; Rebolledo-Zelada, D.; Villarroel, A.; et al. Tolerogenic dendritic cell transfer ameliorates systemic lupus erythematosus in mice. Immunology 2019, 158, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Funda, D.P.; Goliáš, J.; Hudcovic, T.; Kozáková, H.; Špíšek, R.; Palová-Jelínková, L. Antigen Loading (e.g., Glutamic Acid Decarboxylase 65) of Tolerogenic DCs (tolDCs) Reduces Their Capacity to Prevent Diabetes in the Non-Obese Diabetes (NOD)-Severe Combined Immunodeficiency Model of Adoptive Cotransfer of Diabetes As Well As in NOD Mice. Front. Immunol. 2018, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Paiatto, L.N.; Silva, F.G.D.; Yamada, T.; Tamashiro, W.M.S.C.; Simioni, P.U. Adoptive transfer of dendritic cells expressing CD11c reduces the immunological response associated with experimental colitis in BALB/c mice. PLoS ONE 2018, 13, e0196994. [Google Scholar] [CrossRef] [PubMed]




| TolDC Induction Method | Study Model | Antigen Loading/Maturation Stimulus | Result | References |
|---|---|---|---|---|
| Ex vivo, MoDC differentiation media in combination with 1,25 dihydroxyvitamin D3+ Dexamethazone | In vitro model of MoDCs isolated from SLE patients | Lipopolysaccharide | Induces regulatory T cells and modulates cytokines | [46] |
| Ex vivo, MoDC differentiation media in combination with Rosiglitazone + Dexamethasone | In vitro model of MoDCs isolated from SLE patients | Lipopolysaccharide + Autologous Apoptotic Lymphocytes | Suppresses T cell priming and modulates cytokines | [47] |
| Ex vivo, either MoDC differentiation media Only (GM-CSF+IL-4) or combination with P Selectin or PD-1 or IL-10 | In vitro model of MoDCs isolated from SLE patients | None | Diminished maturation signals expression, increased capability to induce Th17 cells, combination with IL-10 induces most potent regulatory T cells | [48] |
| Ex vivo, MoDC differentiation media in combination with Lactobacillus delbruekii + Lactobacillus rhamnosus | In vitro model of MoDCs isolated from SLE patients | None | Suppresses maturation signal expression, increases IDO and IL-10, and decreases IL-12 | [49] |
| Ex vivo, MoDC differentiation media in combination with Curcumin or Berberin | In vitro model of MoDCs isolated from SLE patients | LPS | Suppresses maturation signal expression, decreases IL-12, increases IL-10 | [50] |
| Ex vivo, MoDC differentiation media in combination with 1,25 dihydroxy vitamin D3 | Mice model of multiple Sclerosis | None | Increase the proportion of regulatory T cells, CD4+IL-10+ T cells, and regulatory B cells in the spleen, reducing the infiltration of Th1 and Th17 cells into the spinal cord | [51] |
| Ex vivo transfection with plasmid lentivirus resulting in excessive expression of 25-hydroxyvitamin D 1α-hydroxylase | Mice model of multiple sclerosis | Myelin Antigens | Induces Th2, Treg 1, and T reg Foxp3+ in peripheral lymphoid tissue, suppressing specific symptoms of myelin | [52] |
| Ex vivo, MoDC differentiation medium Only (GM-CSF+IL-4) | Mice model of T1DM | Absence or antigen of pancreatic β cells | Inhibition of the progression of diabetes symptoms regardless of antigens, increasing proliferation of T regulator cells | [53] |
| TolDC Induction Method | Disease | Antigen Loading/Maturation Stimulus | Administration | Clinical Trial Phase | Result | Challenge |
|---|---|---|---|---|---|---|
| Ex vivo MoDC Autolog | Rheumatoid Arthritis [54] | Autologous synovial fluid | Intraarticular | Ia | No immunomodulating effect or systemic effect. Improved symptoms were obtained in two out of three subjects. | Doses are given based on the protocol used for cancer because there have been no reports of tolDC administration for inflammatory arthritis. In addition, whether the intraarticular route can trigger systemic tolerance effects is unknown. Finally, the intraarticular route is invasive, making it undesirable for multiple dosages. |
| Ex vivo MoDC Autolog | T1DM [55] | None | Intradermal | I | Safe and well-tolerated | It cannot induce a systemic immunosuppressive response. |
| Ex vivo MoDC Autolog | Multiple sclerosis+Neuromyelitis Optica [56] | Some Myelin Proteins +AQP4 | Intravenous | Ib | Safe and well tolerated. Increased Treg1 and anti-inflammatory cytokines (IL-10) were obtained. | The specific antigen target in multiple sclerosis is still unknown. |
| Ex vivo MoDC Autolog | T1DM [57] | Proinsulin peptide C19-A3 | Intradermal | I | Safe and well tolerated. Induces an immune tolerance response for up to 3 years, a transient decrease in CD4+ and CD8+ T cell responses to pancreatic islet cell autoantigens, and increases T reg cells and memory CD4+ T cells after the first injection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonny; Sitepu, E.C.; Nidom, C.A.; Wirjopranoto, S.; Sudiana, I.K.; Ansori, A.N.M.; Putranto, T.A. Ex Vivo-Generated Tolerogenic Dendritic Cells: Hope for a Definitive Therapy of Autoimmune Diseases. Curr. Issues Mol. Biol. 2024, 46, 4035-4048. https://doi.org/10.3390/cimb46050249
Jonny, Sitepu EC, Nidom CA, Wirjopranoto S, Sudiana IK, Ansori ANM, Putranto TA. Ex Vivo-Generated Tolerogenic Dendritic Cells: Hope for a Definitive Therapy of Autoimmune Diseases. Current Issues in Molecular Biology. 2024; 46(5):4035-4048. https://doi.org/10.3390/cimb46050249
Chicago/Turabian StyleJonny, Enda Cindylosa Sitepu, Chairul A. Nidom, Soetojo Wirjopranoto, I. Ketut Sudiana, Arif N. M. Ansori, and Terawan Agus Putranto. 2024. "Ex Vivo-Generated Tolerogenic Dendritic Cells: Hope for a Definitive Therapy of Autoimmune Diseases" Current Issues in Molecular Biology 46, no. 5: 4035-4048. https://doi.org/10.3390/cimb46050249