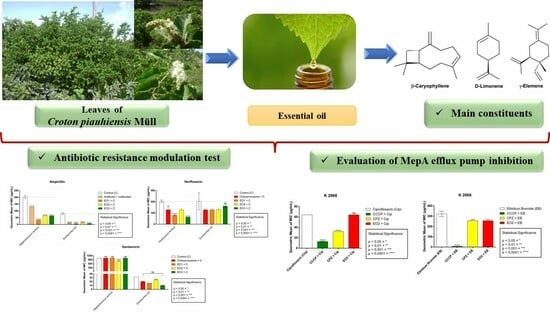
Chemical Composition, Antibacterial and Inhibitory Activity of the Efflux Pump of Essential Oils from Croton piauhiensis Müll.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Material and Essential Oil Extraction
2.3. Bacterial Strains
2.4. Drugs
2.5. Antibacterial Activity
2.6. Antibiotic Resistance Modulation Test
2.7. Evaluation of MepA Efflux Pump Inhibition
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition of Essential Oil
3.2. Direct Antibacterial Activity by Minimum Inhibitory Concentration (MIC)
3.3. Modulatory-Antibiotic Activity of EOCP
3.4. Efflux Pump in Staphylococcus aureus K2068, Carrier of the MepA Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Guschin, A.; Ryzhikh, P.; Rumyantseva, T.; Gomberg, M.; Unemo, M. Treatment efficacy, treatment failures and selection of macrolide resistance in patients with high load of Mycoplasma genitalium during treatment of male urethritis with josamycin. BMC Infect. Dis. 2015, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Z.L.; Guo, J.H.; de la Fuente-Nunez, C.; Wang, J.Q.; Han, B.; Tao, H.; Liu, J.; Wang, X.M. Bacterial resistance to antibacterial agents: Mechanisms, control strategies, and implications for global health. Sci. Total Environ. 2023, 860, 160461. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Lima, M.C.; Paiva de Sousa, C.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef]
- Dawood, M.A.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.; Alagawany, M.; Kari, Z.A.; Abdul Razab, M.K.A.; Hamid, N.K.A.; Moonmanee, T.; Van Doan, H. Exploring the roles of dietary herbal essential oils in aquaculture: A review. Animals 2022, 12, 823. [Google Scholar] [CrossRef]
- Dias-Souza, M.V.; Perpétuo, A.A.; dos Santos, G.S.; Machado, L.F.C.; dos Santos, R.M. Natural products in drug discovery: Meeting the urgency for new antimicrobials for human and veterinary use. AIMS Mol. Sci. 2023, 10, 11–21. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Mayers, D. Antimicrobial Drug Resistance Clinical and Epidemiological Aspects; Humana Press: Totowa, NJ, USA, 2009; Volume 2. [Google Scholar]
- Garoy, E.Y.; Gebreab, Y.B.; Achila, O.O.; Tekeste, D.G.; Kesete, R.; Ghirmay, R.; Kiflay, R.; Tesfu, T. Methicillin-Resistant Staphylococcus aureus (MRSA): Prevalence and Antimicrobial Sensitivity Pattern among Patients—A Multicenter Study in Asmara, Eritrea. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 8321834. [Google Scholar] [CrossRef]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 2019, 149, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Jang, S. Multidrug efflux pumps in Staphylococcus aureus and their clinical implications. J. Microbiol. 2016, 54, 1–8. [Google Scholar] [CrossRef]
- Lamut, A.; Peterlin Mašič, L.; Kikelj, D.; Tomašič, T. Efflux pump inhibitors of clinically relevant multidrug resistant bacteria. Med. Res. Rev. 2019, 39, 2460–2504. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Ganjloo, S.; Pourmand, M.R.; Mashhadi, R.; Ghazvini, K. Epidemiology of efflux pumps genes mediating resistance among Staphylococcus aureus: A systematic review. Microb. Pathog. 2020, 139, 103850. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.W.; Bezerra, P.D.N.; De Oliveira, M.S.; Da Costa, W.A.; Ferreira, G.C.; De Carvalho, R.N. Bioactive Compounds and Biological Activity of Croton Species (Euphorbiaceae): An Overview. Curr. Bioact. Compd. 2020, 16, 383–393. [Google Scholar] [CrossRef]
- Rosa, M.S.C.S.; Mendonça-Filho, R.R.; Bizzo, H.R.; Rodrigues, I.A.; Soares, R.M.A.; Souto-Padrón, T.; Alviano, C.S.; Lopes, A.H.C.S. Antileishmanial activity of linalool-rich essential oil from Croton cajucara Benth. Antimicrob. Agents Chemother. 2003, 47, 1895–1901. [Google Scholar] [CrossRef]
- Guimarães, D.O.; Momesso, L.d.S.; Pupo, M.T. Antibióticos: Importância terapêutica e perspectivas para a descoberta e desenvolvimento de novos agentes. Química Nova 2010, 33, 667–679. [Google Scholar] [CrossRef]
- da Silva, M.M.C.; Neto, J.B.D.; dos Santos, A.T.L.; Oliveira-Tintino, C.D.D.; de Araújo, A.C.J.; Freitas, P.R.; da Silva, L.E.; do Amaral, W.; Deschamps, C.; de Azevedo, F.R.; et al. Antibiotic-Potentiating Activity of the Schinus terebinthifolius Raddi Essential Oil against MDR Bacterial Strains. Plants 2023, 12, 1587. [Google Scholar] [CrossRef]
- Tulgar, S.; Alasehir, E.A.; Selvi, O. The antimicrobial activity of ephedrine and admixture of ephedrine and propofol: An in vitro study. Braz. J. Anesthesiol. (Engl. Ed.) 2018, 68, 69–74. [Google Scholar] [CrossRef]
- Sobrinho, A.C.; Souza, E.; Marcos, F.; Maria, R.; Paulo, N.; Lcio, S.; Morais, S.; Raquel, O.; Carolina, S. Cytotoxicity, antifungal and antioxidant activities of the essential oil from Eupatorium ballotifolium Kunth (Asteraceae). Afr. J. Pharm. Pharmacol. 2016, 10, 346–355. [Google Scholar] [CrossRef]
- Escobar, J.D.; Prieto, C.; Pardo-Figuerez, M.; Lagaron, J.M. Dragon’s Blood Sap: Storage Stability and Antioxidant Activity. Molecules 2018, 23, 2641. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Tudu, A.K. Tackling multidrug-resistant Staphylococcus aureus by natural products and their analogues acting as NorA efflux pump inhibitors. Bioorganic Med. Chem. 2023, 80, 117187. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.D.; da Rocha, W.R.V.; Rodrigues, J.F.B.; Alves, H.D. Synergistic and Antibiofilm Effects of the Essential Oil from Croton conduplicatus (Euphorbiaceae) against Methicillin-Resistant Staphylococcus aureus. Pharmaceuticals 2023, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Zago, J.A.A.; Ushimaru, P.I.; Barbosa, L.N.; Fernandes, A., Jr. Sinergismo entre óleos essenciais e drogas antimicrobianas sobre linhagens de Staphylococcus aureus e Escherichia coli isoladas de casos clínicos humanos. Rev. Bras. Farmacogn. 2009, 19, 828–833. [Google Scholar] [CrossRef]
- Morais, S.M.d.; Catunda Júnior, F.E.A.; Silva, A.R.A.d.; Martins Neto, J.S.; Rondina, D.; Cardoso, J.H.L. Atividade antioxidante de óleos essenciais de espécies de Croton do nordeste do Brasil. Química Nova 2006, 29, 907–910. [Google Scholar] [CrossRef]
- da Silva Almeida, J.R.G.; de Souza, A.V.V.; de Oliveira, A.P.; dos Santos, U.S.; de Souza, M.D.; Bispo, L.P.; Turatti, I.C.C.; Lopes, N.P. Chemical composition of essential oils from the stem barks of Croton conduplicatus (Euphorbiaceae) native to the Caatinga biome. Afr. J. Pharm. Pharmacol. 2015, 9, 98–101. [Google Scholar] [CrossRef]
- Xu, W.-H.; Liu, W.-Y.; Liang, Q. Chemical Constituents from Croton Species and Their Biological Activities. Molecules 2018, 23, 2333. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- German, N.; Wei, P.; Kaatz, G.W.; Kerns, R.J. Synthesis and evaluation of fluoroquinolone derivatives as substrate-based inhibitors of bacterial efflux pumps. Eur. J. Med. Chem. 2008, 43, 2453–2463. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Javadpour, M.M.; Juban, M.M.; Lo, W.-C.J.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De Novo Antimicrobial Peptides with Low Mammalian Cell Toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Kadri, S.S. Key Takeaways from the U.S. CDC’s 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit. Care Med. 2020, 48, 939–945. [Google Scholar] [CrossRef]
- Freitas, T.S.; Campina, F.F.; Costa, M.S.; Rocha, J.E.; Cruz, R.P.; Pinheiro, J.C.A.; Pereira-Júnior, F.N.; Lima, M.A.; Pires de Sá, M.s.F.C.; Teixeira, A.M.R.; et al. UPLC-QTOF-MS/MS analysis and antibacterial activity of the Manilkara zapota (L.) P. Royen against Escherichia coli and other MDR bacteria. Cell. Mol. Biol. 2021, 67, 116–124. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Bonilla, O.H.; Lucena, E.M.P. Influência da sazonalidade e do ciclo circadiano no rendimento e composição química dos óleos essenciais de Croton spp. da Caatinga. Iheringia Série Botânica 2018, 73, 31–38. [Google Scholar] [CrossRef]
- Sefidkon, F.; Abbasi, K.; Jamzad, Z.; Ahmadi, S. The effect of distillation methods and stage of plant growth on the essential oil content and composition of Satureja rechingeri Jamzad. Food Chem. 2007, 100, 1054–1058. [Google Scholar] [CrossRef]
- Cerqueira, M.D.d.; Marques, E.d.J.; Martins, D.; Roque, N.F.; Cruz, F.G.; Guedes, M.L.d.S. Variação sazonal da composição do óleo essencial de Myrcia salzmannii Berg. (Myrtaceae). Química Nova 2009, 32, 1544–1548. [Google Scholar] [CrossRef]
- Houghton, P.J.; Howes, M.J.; Lee, C.C.; Steventon, G. Uses and abuses of in vitro tests in ethnopharmacology: Visualizing an elephant. J. Ethnopharmacol. 2007, 110, 391–400. [Google Scholar] [CrossRef]
- Baser, K.H.; Demirci, B.; Iscan, G.; Hashimoto, T.; Demirci, F.; Noma, Y.; Asakawa, Y. The essential oil constituents and antimicrobial activity of Anthemis aciphylla BOISS. var. discoidea BOISS. Chem. Pharm. Bull. 2006, 54, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Moo, C.-L.; Yang, S.-K.; Osman, M.-A.; Yuswan, M.H.; Loh, J.-Y.; Lim, W.-M.; Lim, S.-H.-E.; Lai, K.-S. Antibacterial Activity and Mode of Action of β-caryophyllene on. Pol. J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 7773. [Google Scholar] [CrossRef]
- Mingeot-Leclercq, M.-P.; Glupczynski, Y.; Tulkens Paul, M. Aminoglycosides: Activity and Resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [CrossRef]
- Jana, S.; Deb, J.K. Molecular understanding of aminoglycoside action and resistance. Appl. Microbiol. Biotechnol. 2006, 70, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Z.Q.; Zheng, P.Y.; Tang, F.A.; Yang, P.C. Influence of efflux pump inhibitors on the multidrug resistance of Helicobacter pylori. World J. Gastroenterol. 2010, 16, 1279–1284. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gaur, R.; Sharma, A.; Akther, J.; Saini, M.; Bhakuni, R.S.; Pathania, R. A novel bi-functional chalcone inhibits multi-drug resistant Staphylococcus aureus and potentiates the activity of fluoroquinolones. Bioorg. Chem. 2019, 83, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.L.A.B.; Bezerra, C.F.; Confortin, C.; da Silva, L.E.; Marinho, E.M.; Marinho, M.M.; Vasconcelos, M.A.; da Silva, T.G.; Marinho, E.S.; Teixeira, A.M.R.; et al. Chemical composition and potentiating action of Norfloxacin mediated by the essential oil of Piper caldense C.D.C. against Staphylococcus aureus strains overexpressing efflux pump genes. Arch. Microbiol. 2021, 203, 4727–4736. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Misra, A.; Banerjee, S.; Dam, B. Adaptation of ethidium bromide fluorescence assay to monitor activity of efflux pumps in bacterial pure cultures or mixed population from environmental samples. J. King Saud Univ. Sci. 2020, 32, 939–945. [Google Scholar] [CrossRef]
- Faillace, M.S.; Alves Borges Leal, A.L.; Araújo de Oliveira Alcântara, F.; Ferreira, J.H.L.; de Siqueira-Júnior, J.P.; Sampaio Nogueira, C.E.; Barreto, H.M.; Peláez, W.J. Inhibition of the NorA efflux pump of S. aureus by (Z)-5-(4-Fluorobenzylidene)-Imidazolidines. Bioorg. Med. Chem. Lett. 2021, 31, 127670. [Google Scholar] [CrossRef]







| Compounds | KI * | Percent Composition (%) | ||
|---|---|---|---|---|
| 6 h | 12 h | 18 h | ||
| α-Thujene | 930 | 0.40 | 0.19 | 0.49 |
| α-Pinene | 939 | 2.95 | 1.59 | 2.84 |
| Camphene | 954 | 0.12 | 0.13 | |
| β-Pinene | 979 | 0.28 | ||
| β-Myrcene | 990 | 3.55 | 2.19 | 3.00 |
| α-Phellandrene | 1002 | 0.44 | 0.77 | 0.77 |
| 2-Carene | 1002 | 1.16 | ||
| (+)-4-Carene | 1011 | 0.91 | ||
| α-terpinene | 1017 | 0.27 | 0.22 | 0.36 |
| p-Cymene | 1024 | 4.04 | 2.08 | 2.70 |
| β-Phellandrene | 1029 | 0.34 | 0.56 | 1.00 |
| D-Limonene | 1029 | 13.27 | 9.14 | 15.95 |
| Eucalyptol | 1031 | 2.91 | 2.77 | 1.98 |
| β-Ocimene | 1037 | 1.12 | 0.72 | 1.08 |
| γ-Terpinene | 1059 | 5.56 | 3.68 | 4.60 |
| Terpinolene | 1088 | 1.45 | ||
| Linalool | 1096 | 1.18 | 1.16 | 1.24 |
| Terpinen-4-ol | 1177 | 0.63 | 0.74 | 1.01 |
| α-Terpineol | 1188 | 0.35 | 0.37 | 0.38 |
| Isoamyl tiglate (E) | 1192 | 0.14 | 0.15 | 0.15 |
| Hexenyl valerate (Z) | 1281 | 0.39 | 0.20 | 0.27 |
| Bornyl acetate | 1285 | 0.11 | ||
| Cycloisolongifolene | 1319 | 0.34 | 0.18 | |
| α-Terpinyl acetate | 1349 | 0.20 | 0.18 | 0.27 |
| Cyclosativene | 1371 | 0.11 | ||
| α-Copaene | 1376 | 1.06 | 1.01 | 0.82 |
| Isoledene | 1376 | 0.22 | 0.27 | 0.20 |
| β-Damascenone | 1384 | 0.42 | 0.34 | |
| b-Cubebene | 1388 | 0.13 | ||
| b-Elemene | 1390 | 2.10 | 2.43 | 1.66 |
| Cyperene | 1398 | 0.18 | ||
| β-Caryophyllene | 1419 | 21.23 | 22.86 | 16.95 |
| β-copaene | 1432 | 8.28 | 10.07 | 7.70 |
| β-Gurjunene | 1433 | 0.62 | 0.61 | 0.48 |
| γ-Elemene | 1436 | 6.59 | 12.61 | 9.59 |
| Aromandendrene | 1441 | 0.40 | 2.55 | 1.41 |
| α-Humulene | 1454 | 2.70 | 2.85 | 2.17 |
| γ-Muurolene | 1479 | 0.46 | 0.36 | 0.51 |
| γ-Himachalene | 1482 | 0.12 | ||
| α-Amorphene | 1484 | 0.11 | 0.59 | 0.19 |
| Valencene | 1496 | 0.06 | ||
| α-Muurolene | 1500 | 0.37 | 0.57 | 0.42 |
| Cuparene | 1504 | 0.16 | ||
| δ-Cadinene | 1523 | 2.18 | 2.83 | 2.13 |
| Cadina-1,4-diene (trans) | 1534 | 0.16 | 0.29 | 0.17 |
| Germacrene B | 1561 | 0.23 | 0.32 | |
| Palustrol | 1568 | 0.25 | 0.21 | 0.27 |
| Spathulenol | 1578 | 3.08 | 2.5 | 2.59 |
| Caryophyllene oxide | 1583 | 0.11 | 0.10 | |
| Gleenol | 1587 | 0.09 | ||
| Viridiflorol | 1592 | 0.79 | ||
| Ledol | 1602 | 0.28 | 0.35 | |
| α-epi-Muurolol | 1642 | 0.15 | 0.67 | 4.03 |
| Cubenol | 1646 | 0.32 | 0.33 | 0.34 |
| α-Cadinol | 1654 | 3.92 | 3.95 | 1.22 |
| Selin-11-en-4-α-ol | 1659 | 0.14 | ||
| Cembrene | 1938 | 0.23 | ||
| Elemol | 1549 | 0.11 | ||
| Pentadecanone | 1697 | 0.17 | 0.19 | 0.19 |
| Phytol | 1943 | 0.23 | 0.35 | 0.30 |
| Total | 96.04 | 96.82 | 96.25 | |
| Strains | EOCP (µg/mL) | ||
|---|---|---|---|
| 6 h | 12 h | 18 h | |
| MIC | MIC | MIC | |
| Staphylococcus aureus 10—SA10 | 813 | 256 | ≥1024 |
| Staphylococcus aureus ATCC 25923—SA ATCC | ≥1024 | 25 | 645 |
| Escherichia coli 06—EC06 | ≥1024 | 323 | ≥1024 |
| Escherichia coli ATCC 25922—EC ATCC | 406 | 128 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, B.G.; de Freitas, T.S.; Costa, M.d.S.; da Silva, A.R.P.; Coutinho, H.D.M.; de Morais, S.M.; Marinho, E.S.; Teixeira, A.M.R.; Silva dos Santos, H. Chemical Composition, Antibacterial and Inhibitory Activity of the Efflux Pump of Essential Oils from Croton piauhiensis Müll. Nutraceuticals 2023, 3, 591-604. https://doi.org/10.3390/nutraceuticals3040042
Cruz BG, de Freitas TS, Costa MdS, da Silva ARP, Coutinho HDM, de Morais SM, Marinho ES, Teixeira AMR, Silva dos Santos H. Chemical Composition, Antibacterial and Inhibitory Activity of the Efflux Pump of Essential Oils from Croton piauhiensis Müll. Nutraceuticals. 2023; 3(4):591-604. https://doi.org/10.3390/nutraceuticals3040042
Chicago/Turabian StyleCruz, Beatriz Gonçalves, Thiago Sampaio de Freitas, Maria do Socorro Costa, Ana Raquel Pereira da Silva, Henrique Douglas Melo Coutinho, Selene Maia de Morais, Emmanuel Silva Marinho, Alexandre Magno Rodrigues Teixeira, and Hélcio Silva dos Santos. 2023. "Chemical Composition, Antibacterial and Inhibitory Activity of the Efflux Pump of Essential Oils from Croton piauhiensis Müll." Nutraceuticals 3, no. 4: 591-604. https://doi.org/10.3390/nutraceuticals3040042