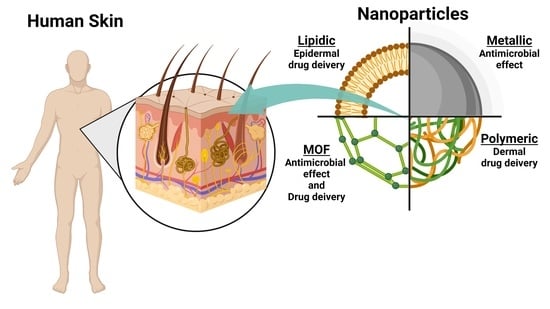
The Human Dermis as a Target of Nanoparticles for Treating Skin Conditions
Abstract
:1. Skin Function and Composition
2. Dermis
3. The Cellular Population of the Dermis
3.1. Fibroblast
3.2. Immune Cells
4. Dermis as a Target for Nanotechnology-Based Treatments
4.1. Skin Penetration of Nanoparticles
4.2. Effect of Nanoparticles over the Dermis
4.2.1. Inorganic Nanoparticles
4.2.2. Polymeric-Based Nanoparticles
4.2.3. Lipid-Based Nanoparticles
5. Metal-Organic Frameworks as an Integrative Tool for Skin Treatments
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, Y.Z.; Simmons, L.W.; Rhodes, G. Predictors of facial attractiveness and health in humans. Sci. Rep. 2017, 7, 39731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seth, D.; Cheldize, K.; Brown, D.; Freeman, E.F. Global Burden of Skin Disease: Inequities and Innovations. Curr. Derm. Rep. 2017, 6, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Collins, S.A.B.; Resneck, J.S., Jr.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. The burden of skin disease in the United States. J. Am. Acad. Derm. 2017, 76, 958–972.e952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haydont, V.; Bernard, B.A.; Fortunel, N.O. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech. Ageing Dev. 2019, 177, 150–156. [Google Scholar] [CrossRef]
- Eyerich, S.; Eyerich, K.; Traidl-Hoffmann, C.; Biedermann, T. Cutaneous Barriers and Skin Immunity: Differentiating A Connected Network. Trends Immunol. 2018, 39, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Derm. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Horsley, V. Skin in the Game: Stem Cells in Repair, Cancer, and Homeostasis. Cell 2020, 181, 492–494. [Google Scholar] [CrossRef]
- Fisher, G.J.; Shao, Y.; He, T.; Qin, Z.; Perry, D.; Voorhees, J.J.; Quan, T. Reduction of fibroblast size/mechanical force down-regulates TGF-beta type II receptor: Implications for human skin aging. Aging Cell 2016, 15, 67–76. [Google Scholar] [CrossRef]
- Janson, D.G.; Saintigny, G.; van Adrichem, A.; Mahe, C.; El Ghalbzouri, A. Different gene expression patterns in human papillary and reticular fibroblasts. J. Investig. Derm. 2012, 132, 2565–2572. [Google Scholar] [CrossRef]
- Dengjel, J.; Bruckner-Tuderman, L.; Nystrom, A. Skin proteomics—Analysis of the extracellular matrix in health and disease. Expert Rev. Proteom. 2020, 17, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Abdo, H.; Calvo-Enrique, L.; Lopez, J.M.; Song, J.; Zhang, M.D.; Usoskin, D.; El Manira, A.; Adameyko, I.; Hjerling-Leffler, J.; Ernfors, P. Specialized cutaneous Schwann cells initiate pain sensation. Science 2019, 365, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.A.; Licup, A.J.; Sharma, A.; Rens, R.; MacKintosh, F.C.; Koenderink, G.H. The Role of Network Architecture in Collagen Mechanics. Biophys. J. 2018, 114, 2665–2678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [Green Version]
- Thulabandu, V.; Chen, D.; Atit, R.P. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e307. [Google Scholar] [CrossRef]
- Griffin, M.F.; desJardins-Park, H.E.; Mascharak, S.; Borrelli, M.R.; Longaker, M.T. Understanding the impact of fibroblast heterogeneity on skin fibrosis. Dis. Model. Mech. 2020, 13, dmm044164. [Google Scholar] [CrossRef]
- Driskell, R.R.; Watt, F.M. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015, 25, 92–99. [Google Scholar] [CrossRef]
- Hu, M.S.; Moore, A.L.; Longaker, M.T. A Fibroblast Is Not a Fibroblast Is Not a Fibroblast. J. Investig. Derm. 2018, 138, 729–730. [Google Scholar] [CrossRef] [Green Version]
- Philippeos, C.; Telerman, S.B.; Oules, B.; Pisco, A.O.; Shaw, T.J.; Elgueta, R.; Lombardi, G.; Driskell, R.R.; Soldin, M.; Lynch, M.D.; et al. Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations. J. Investig. Derm. 2018, 138, 811–825. [Google Scholar] [CrossRef] [Green Version]
- Korosec, A.; Frech, S.; Gesslbauer, B.; Vierhapper, M.; Radtke, C.; Petzelbauer, P.; Lichtenberger, B.M. Lineage Identity and Location within the Dermis Determine the Function of Papillary and Reticular Fibroblasts in Human Skin. J. Investig. Derm. 2019, 139, 342–351. [Google Scholar] [CrossRef]
- Nauroy, P.; Barruche, V.; Marchand, L.; Nindorera-Badara, S.; Bordes, S.; Closs, B.; Ruggiero, F. Human Dermal Fibroblast Subpopulations Display Distinct Gene Signatures Related to Cell Behaviors and Matrisome. J. Investig. Derm. 2017, 137, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Korosec, A.; Frech, S.; Lichtenberger, B.M. Isolation of Papillary and Reticular Fibroblasts from Human Skin by Fluorescence-activated Cell Sorting. J. Vis. Exp. 2019, 147, e59372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karppinen, S.M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research 2019, 8, 787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, H.; Zhang, T.; Fan, J.; Xiao, R. The Superficial Dermis May Initiate Keloid Formation: Histological Analysis of the Keloid Dermis at Different Depths. Front. Physiol. 2017, 8, 885. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.C.; Hu, Y.F.; Zhu, D.H.; Cheng, Q.; Gu, J.J.; Feng, Q.L.; Zhang, L.X.; Xu, Y.P.; Wang, D.; Rong, Z.; et al. Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nat. Commun. 2021, 12, 3709. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; McCulloch, C.A.; Coelho, N.M. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Exp. Cell Res. 2019, 379, 119–128. [Google Scholar] [CrossRef]
- Huang, X.; Gu, S.; Liu, C.; Zhang, L.; Zhang, Z.; Zhao, Y.; Khoong, Y.; Li, H.; Gao, Y.; Liu, Y.; et al. CD39(+) Fibroblasts Enhance Myofibroblast Activation by Promoting IL-11 Secretion in Hypertrophic Scars. J. Investig. Derm. 2022, 142, 1065–1076.e1019. [Google Scholar] [CrossRef]
- Gyorfi, A.H.; Matei, A.E.; Fuchs, M.; Liang, C.; Rigau, A.R.; Hong, X.; Zhu, H.; Luber, M.; Bergmann, C.; Dees, C.; et al. Engrailed 1 coordinates cytoskeletal reorganization to induce myofibroblast differentiation. J. Exp. Med. 2021, 218, e20201916. [Google Scholar] [CrossRef]
- Mascharak, S.; desJardins-Park, H.E.; Davitt, M.F.; Griffin, M.; Borrelli, M.R.; Moore, A.L.; Chen, K.; Duoto, B.; Chinta, M.; Foster, D.S.; et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 2021, 372, eaba2374. [Google Scholar] [CrossRef]
- Montoni, A.; George, K.M.; Soeur, J.; Tran, C.; Marrot, L.; Rochette, P.J. Chronic UVA1 Irradiation of Human Dermal Fibroblasts: Persistence of DNA Damage and Validation of a Cell Cultured-Based Model of Photoaging. J. Investig. Derm. 2019, 139, 1821–1824.e1823. [Google Scholar] [CrossRef]
- Birch-Machin, M.A.; Russell, E.V.; Latimer, J.A. Mitochondrial DNA damage as a biomarker for ultraviolet radiation exposure and oxidative stress. Br. J. Derm. 2013, 169 (Suppl. S2), 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Weinberger, B.; Arnold, C.R.; Maier, A.B.; Westendorp, R.G.; Grubeck-Loebenstein, B. The effect of chronological age on the inflammatory response of human fibroblasts. Exp. Gerontol. 2012, 47, 749–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavinato, M.; Jansen-Durr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kenny, F.N.; Drymoussi, Z.; Delaine-Smith, R.; Kao, A.P.; Laly, A.C.; Knight, M.M.; Philpott, M.P.; Connelly, J.T. Tissue stiffening promotes keratinocyte proliferation through activation of epidermal growth factor signaling. J. Cell Sci. 2018, 131, jcs.215780. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.K.; Yuan, W.; Mori, Y.; Varga, J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-beta involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene 2000, 19, 3546–3555. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Qian, Y.; Jin, R.; Wo, Y.; Chen, J.; Wang, C.; Wang, D. Effects of TRAP-1-like protein (TLP) gene on collagen synthesis induced by TGF-beta/Smad signaling in human dermal fibroblasts. PLoS ONE 2013, 8, e55899. [Google Scholar] [CrossRef] [Green Version]
- Strnadova, K.; Sandera, V.; Dvorankova, B.; Kodet, O.; Duskova, M.; Smetana, K.; Lacina, L. Skin aging: The dermal perspective. Clin. Derm. 2019, 37, 326–335. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Ho, A.W.; Kupper, T.S. T cells and the skin: From protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 2019, 19, 490–502. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Gray, E.E.; Zhang, Y.; Ramirez-Valle, F.; Cyster, J.G. Sphingosine-1-phosphate receptor 2 restrains egress of gammadelta T cells from the skin. J. Exp. Med. 2019, 216, 1487–1496. [Google Scholar] [CrossRef]
- Duffy, D.; Perrin, H.; Abadie, V.; Benhabiles, N.; Boissonnas, A.; Liard, C.; Descours, B.; Reboulleau, D.; Bonduelle, O.; Verrier, B.; et al. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity 2012, 37, 917–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkgraaf, F.E.; Matos, T.R.; Hoogenboezem, M.; Toebes, M.; Vredevoogd, D.W.; Mertz, M.; van den Broek, B.; Song, J.Y.; Teunissen, M.B.M.; Luiten, R.M.; et al. Tissue patrol by resident memory CD8(+) T cells in human skin. Nat. Immunol. 2019, 20, 756–764, Erratum in Nat. Immunol. 2020, 21, 696. [Google Scholar] [CrossRef] [PubMed]
- Jee, M.H.; Mraz, V.; Geisler, C.; Bonefeld, C.M. gammadelta T cells and inflammatory skin diseases. Immunol. Rev. 2020, 298, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Di Nardo, A. Skin neurogenic inflammation. Semin. Immunopathol. 2018, 40, 249–259. [Google Scholar] [CrossRef]
- Lowy, D.B.; Makker, P.G.S.; Moalem-Taylor, G. Cutaneous Neuroimmune Interactions in Peripheral Neuropathic Pain States. Front. Immunol. 2021, 12, 660203. [Google Scholar] [CrossRef]
- Sumpter, T.L.; Balmert, S.C.; Kaplan, D.H. Cutaneous immune responses mediated by dendritic cells and mast cells. JCI Insight 2019, 4, e123947. [Google Scholar] [CrossRef]
- Kuroishi, T.; Bando, K.; Bakti, R.K.; Ouchi, G.; Tanaka, Y.; Sugawara, S. Migratory dendritic cells in skin-draining lymph nodes have nickel-binding capabilities. Sci. Rep. 2020, 10, 5050. [Google Scholar] [CrossRef] [Green Version]
- Ibusuki, A.; Nishikawa, T.; Hiraki, T.; Okano, T.; Imai, K.; Kanegane, H.; Ohnishi, H.; Kato, Z.; Fujii, K.; Tanimoto, A.; et al. Prominent dermal Langerhans cells in an Omenn syndrome patient with a novel mutation in the IL2RG gene. J. Derm. 2019, 46, 1019–1023. [Google Scholar] [CrossRef]
- Bastonini, E.; Bellei, B.; Filoni, A.; Kovacs, D.; Iacovelli, P.; Picardo, M. Involvement of non-melanocytic skin cells in vitiligo. Exp. Derm. 2019, 28, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Lowes, M.A.; Suarez-Farinas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef]
- Krishnan, V.; Mitragotri, S. Nanoparticles for topical drug delivery: Potential for skin cancer treatment. Adv. Drug Deliv. Rev. 2020, 153, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm. Sin. B 2022, 12, 3028–3048. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, C.; Baik, S.; Kim, D.; Hyeon, T.; Kim, D.H. Device-assisted transdermal drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Chu, L.Y.; Burton, S.A.; Hansen, K.J.; Panyam, J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J. Control. Release 2019, 294, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M. Skin penetration/permeation success determinants of nanocarriers: Pursuit of a perfect formulation. Colloids Surf. B Biointerfaces 2021, 203, 111748. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khabir, Z.; Guller, A.E.; Rozova, V.S.; Liang, L.; Lai, Y.J.; Goldys, E.M.; Hu, H.; Vickery, K.; Zvyagin, A.V. Tracing upconversion nanoparticle penetration in human skin. Colloids Surf. B Biointerfaces 2019, 184, 110480. [Google Scholar] [CrossRef]
- Hung, C.F.; Chen, W.Y.; Hsu, C.Y.; Aljuffali, I.A.; Shih, H.C.; Fang, J.Y. Cutaneous penetration of soft nanoparticles via photodamaged skin: Lipid-based and polymer-based nanocarriers for drug delivery. Eur. J. Pharm. Biopharm. 2015, 94, 94–105. [Google Scholar] [CrossRef]
- Hung, C.F.; Fang, C.L.; Al-Suwayeh, S.A.; Yang, S.Y.; Fang, J.Y. Evaluation of drug and sunscreen permeation via skin irradiated with UVA and UVB: Comparisons of normal skin and chronologically aged skin. J. Derm. Sci. 2012, 68, 135–148. [Google Scholar] [CrossRef]
- Richards, G.M.; Oresajo, C.O.; Halder, R.M. Structure and function of ethnic skin and hair. Derm. Clin. 2003, 21, 595–600. [Google Scholar] [CrossRef]
- Salimi, A.; Sharif Makhmal Zadeh, B.; Godazgari, S.; Rahdar, A. Development and Evaluation of Azelaic Acid-Loaded Microemulsion for Transfollicular Drug Delivery Through Guinea Pig Skin: A Mechanistic Study. Adv. Pharm. Bull. 2020, 10, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Otberg, N.; Thiede, G.; Richter, H.; Sterry, W.; Panzner, S.; Lademann, J. Innovative liposomes as a transfollicular drug delivery system: Penetration into porcine hair follicles. J. Investig. Derm. 2006, 126, 1728–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pena-Jimenez, D.; Fontenete, S.; Megias, D.; Fustero-Torre, C.; Grana-Castro, O.; Castellana, D.; Loewe, R.; Perez-Moreno, M. Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. EMBO J. 2019, 38, e101688. [Google Scholar] [CrossRef] [PubMed]
- Try, C.; Moulari, B.; Beduneau, A.; Fantini, O.; Pin, D.; Pellequer, Y.; Lamprecht, A. Size dependent skin penetration of nanoparticles in murine and porcine dermatitis models. Eur. J. Pharm. Biopharm. 2016, 100, 101–108. [Google Scholar] [CrossRef]
- Pelikh, O.; Eckert, R.W.; Pinnapireddy, S.R.; Keck, C.M. Hair follicle targeting with curcumin nanocrystals: Influence of the formulation properties on the penetration efficacy. J. Control. Release 2021, 329, 598–613. [Google Scholar] [CrossRef]
- Busch, L.; Keziban, Y.; Dahne, L.; Keck, C.M.; Meinke, M.C.; Lademann, J.; Patzelt, A. The impact of skin massage frequency on the intrafollicular transport of silica nanoparticles: Validation of the ratchet effect on an ex vivo porcine skin model. Eur. J. Pharm. Biopharm. 2021, 158, 266–272. [Google Scholar] [CrossRef]
- Radtke, M.; Patzelt, A.; Knorr, F.; Lademann, J.; Netz, R.R. Ratchet effect for nanoparticle transport in hair follicles. Eur. J. Pharm. Biopharm. 2017, 116, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Patzelt, A.; Mak, W.C.; Jung, S.; Knorr, F.; Meinke, M.C.; Richter, H.; Ruhl, E.; Cheung, K.Y.; Tran, N.; Lademann, J. Do nanoparticles have a future in dermal drug delivery? J. Control. Release 2017, 246, 174–182. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Meinke, M.C.; Lange-Asschenfeldt, B.; Antoniou, C.; Mak, W.C.; Renneberg, R.; Sterry, W.; Patzelt, A. Drug delivery with topically applied nanoparticles: Science fiction or reality. Skin Pharm. Physiol. 2013, 26, 227–233. [Google Scholar] [CrossRef]
- Kim, M.H.; Jeon, Y.E.; Kang, S.; Lee, J.Y.; Lee, K.W.; Kim, K.T.; Kim, D.D. Lipid Nanoparticles for Enhancing the Physicochemical Stability and Topical Skin Delivery of Orobol. Pharmaceutics 2020, 12, 845. [Google Scholar] [CrossRef]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and Transdermal Drug Delivery Systems: Current and Future Prospects. Drug Deliv. 2006, 13, 175–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-Enabled Transdermal Drug Delivery Systems for Enhanced Dose Control and Tissue Targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Yin, L.; Ma, Z.; Zhang, Y. Chlorogenic Acid-Loaded Mesoporous Silica Nanoparticles Modified with Hexa-Histidine Peptides Reduce Skin Allergies by Capturing Nickel. Molecules 2022, 27, 1430. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Anwer, M.K.; Sartaj, A.; Panda, B.P.; Ali, A.; Zafar, A.; Kumar, V.; Gilani, S.J.; Kala, C.; Taleuzzaman, M. ZnO Nanoparticles of Rubia cordifolia Extract Formulation Developed and Optimized with QbD Application, Considering Ex Vivo Skin Permeation, Antimicrobial and Antioxidant Properties. Molecules 2022, 27, 1450. [Google Scholar] [CrossRef] [PubMed]
- Borges Rosa de Moura, F.; Antonio Ferreira, B.; Helena Muniz, E.; Benatti Justino, A.; Gabriela Silva, A.; de Azambuja Ribeiro, R.I.M.; Oliveira Dantas, N.; Lisboa Ribeiro, D.; de Assis Araujo, F.; Salmen Espindola, F.; et al. Antioxidant, anti-inflammatory, and wound healing effects of topical silver-doped zinc oxide and silver oxide nanocomposites. Int. J. Pharm. 2022, 617, 121620. [Google Scholar] [CrossRef]
- Rao, Y.F.; Chen, W.; Liang, X.G.; Huang, Y.Z.; Miao, J.; Liu, L.; Lou, Y.; Zhang, X.G.; Wang, B.; Tang, R.K.; et al. Epirubicin-loaded superparamagnetic iron-oxide nanoparticles for transdermal delivery: Cancer therapy by circumventing the skin barrier. Small 2015, 11, 239–247. [Google Scholar] [CrossRef]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Fabrication and characterization of PCL/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 356–366. [Google Scholar] [CrossRef]
- Graff, P.; Honzke, S.; Joshi, A.A.; Yealland, G.; Fleige, E.; Unbehauen, M.; Schafer-Korting, M.; Hocke, A.; Haag, R.; Hedtrich, S. Preclinical Testing of Dendritic Core-Multishell Nanoparticles in Inflammatory Skin Equivalents. Mol. Pharm. 2022, 19, 1795–1802. [Google Scholar] [CrossRef]
- Gehrcke, M.; de Bastos Brum, T.; da Rosa, L.S.; Ilha, B.D.; Soares, F.Z.M.; Cruz, L. Incorporation of nanocapsules into gellan gum films: A strategy to improve the stability and prolong the cutaneous release of silibinin. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111624. [Google Scholar] [CrossRef]
- Gupta, T.; Kenjale, P.; Pokharkar, V. QbD-based optimization of raloxifene-loaded cubosomal formulation for transdemal delivery: Ex vivo permeability and in vivo pharmacokinetic studies. Drug Deliv. Transl. Res. 2022, 12, 2979–2992. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Azadi, A.; Daneshamouz, S.; Heidari, R.; Azarpira, N.; Mohammadi-Samani, S. Cyproterone acetate-loaded nanostructured lipid carriers: Effect of particle size on skin penetration and follicular targeting. Pharm. Dev. Technol. 2019, 24, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.K.; Pal, S.; Naoghare, P.K.; Rangasamy, S.; Song, J.M. Shape-Dependent Skin Penetration of Silver Nanoparticles: Does It Really Matter? Sci. Rep. 2015, 5, 16908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jingge, M.; Chengtie, W. Bioactive inorganic particles-based biomaterials for skin tissue engineering. Exploration 2022, 2, 20210083. [Google Scholar] [CrossRef]
- Muchova, J.; Hearnden, V.; Michlovska, L.; Vistejnova, L.; Zavadakova, A.; Smerkova, K.; Kociova, S.; Adam, V.; Kopel, P.; Vojtova, L. Mutual influence of selenium nanoparticles and FGF2-STAB((R)) on biocompatible properties of collagen/chitosan 3D scaffolds: In vitro and ex ovo evaluation. J. Nanobiotechnol. 2021, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X. Gold nanoparticles for skin drug delivery. Int. J. Pharm. 2022, 625, 122122. [Google Scholar] [CrossRef]
- Niu, J.; Chu, Y.; Huang, Y.F.; Chong, Y.S.; Jiang, Z.H.; Mao, Z.W.; Peng, L.H.; Gao, J.Q. Transdermal Gene Delivery by Functional Peptide-Conjugated Cationic Gold Nanoparticle Reverses the Progression and Metastasis of Cutaneous Melanoma. ACS Appl. Mater. Interfaces 2017, 9, 9388–9401. [Google Scholar] [CrossRef]
- Ramadan, S.; Guo, L.; Li, Y.; Yan, B.; Lu, W. Hollow copper sulfide nanoparticle-mediated transdermal drug delivery. Small 2012, 8, 3143–3150. [Google Scholar] [CrossRef] [Green Version]
- Sivasankarapillai, V.S.; Vishnu Kirthi, A.; Akksadha, M.; Indu, S.; Dhiviya Dharshini, U.; Pushpamalar, J.; Karthik, L. Recent advancements in the applications of carbon nanodots: Exploring the rising star of nanotechnology. Nanoscale Adv. 2020, 2, 1760–1773. [Google Scholar] [CrossRef] [Green Version]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Roy, M.; Goswami, P.; Roy, S.; Das, A.K.; Ghosh, S.K.; Dhara, S. Carbon nanodot decorated acellular dermal matrix hydrogel augments chronic wound closure. J. Mater. Chem. B 2020, 8, 9277–9294. [Google Scholar] [CrossRef]
- Zielinska, A.; Carreiro, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Mittal, A.; Raber, A.S.; Schaefer, U.F.; Weissmann, S.; Ebensen, T.; Schulze, K.; Guzman, C.A.; Lehr, C.M.; Hansen, S. Non-invasive delivery of nanoparticles to hair follicles: A perspective for transcutaneous immunization. Vaccine 2013, 31, 3442–3451. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, W.; Deng, J.; Kan, J.; Guo, T.; Wang, B.; Hao, S. Recombinant Human Hair Keratin Nanoparticles Accelerate Dermal Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 18681–18690. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, C.; Chen, M.; Xi, Y.; Cheng, W.; Mao, C.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; et al. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano 2019, 13, 10279–10293. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Paredes, A.J.; Volpe-Zanutto, F.; Anjani, Q.K.; Utomo, E.; Donnelly, R.F. Dissolving microneedle-mediated dermal delivery of itraconazole nanocrystals for improved treatment of cutaneous candidiasis. Eur. J. Pharm. Biopharm. 2020, 154, 50–61. [Google Scholar] [CrossRef]
- Jin, N.; Pyo, S.M.; Keck, C.M.; Muller, R.H. Azithromycin nanocrystals for dermal prevention of tick bite infections. Pharmazie 2019, 74, 277–285. [Google Scholar] [CrossRef]
- Jeong, H.; Nam, S.; Song, J.; Park, S. Synthesis and physicochemical properties of pH-sensitive hydrogel based on carboxymethyl chitosan/2-hydroxyethyl acrylate for transdermal delivery of nobiletin. J. Drug Deliv. Sci. Technol. 2019, 51, 194–203. [Google Scholar] [CrossRef]
- Junior, D.M.; Hausen, M.A.; Asami, J.; Higa, A.M.; Leite, F.L.; Mambrini, G.P.; Rossi, A.L.; Komatsu, D.; Duek, E.A.R. A New Dermal Substitute Containing Polyvinyl Alcohol with Silver Nanoparticles and Collagen with Hyaluronic Acid: In Vitro and In Vivo Approaches. Antibiotics 2021, 10, 742. [Google Scholar] [CrossRef]
- Kanemaru, M.; Asai, J.; Jo, J.I.; Arita, T.; Kawai-Ohnishi, M.; Tsutsumi, M.; Wada, M.; Tabata, Y.; Katoh, N. Nanoparticle-mediated local delivery of pioglitazone attenuates bleomycin-induced skin fibrosis. J. Derm. Sci. 2019, 93, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Lee, S.; Ki, C.S. Modular formation of hyaluronic acid/beta-glucan hybrid nanogels for topical dermal delivery targeting skin dendritic cells. Carbohydr. Polym. 2021, 252, 117132. [Google Scholar] [CrossRef]
- Yan, Y.; Liang, H.; Liu, X.; Liu, L.; Chen, Y. Topical cationic hairy particles targeting cell free DNA in dermis enhance treatment of psoriasis. Biomaterials 2021, 276, 121027. [Google Scholar] [CrossRef]
- Lorenzoni, R.; Contri, R.V.; Lima, C.K.F.; Barreto, F.; Araujo, B.V.; Pohlmann, A.R.; de Miranda, A.L.P.; Costa, T.D.; Guterres, S.S. Dermatopharmacokinetic and pharmacodynamic evaluation of a novel nanostructured formulation containing capsaicinoids for treating neuropathic pain. Int. J. Pharm. 2021, 596, 120294. [Google Scholar] [CrossRef] [PubMed]
- Sanad, R.A.; Abdel-Bar, H.M. Chitosan-hyaluronic acid composite sponge scaffold enriched with Andrographolide-loaded lipid nanoparticles for enhanced wound healing. Carbohydr. Polym. 2017, 173, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Widjaya, A.S.; Liu, J.; Liu, X.; Long, Z.; Jiang, Y. Cell-penetrating corosolic acid liposome as a functional carrier for delivering chemotherapeutic drugs. Acta Biomater. 2020, 106, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Beaulac, C.; Sachetelli, S.; Lagace, J. In-vitro bactericidal efficacy of sub-MIC concentrations of liposome-encapsulated antibiotic against gram-negative and gram-positive bacteria. J. Antimicrob. Chemother. 1998, 41, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khater, D.; Nsairat, H.; Odeh, F.; Saleh, M.; Jaber, A.; Alshaer, W.; Bawab, A.; Mubarak, M. Design, Preparation, and Characterization of Effective Dermal and Transdermal Lipid Nanoparticles: A Review. Cosmetics 2021, 8, 39. [Google Scholar] [CrossRef]
- Lebron, J.A.; Lopez-Lopez, M.; Garcia-Calderon, C.B.; Rosado, V.I.; Balestra, F.R.; Huertas, P.; Rodik, R.V.; Kalchenko, V.I.; Bernal, E.; Moya, M.L.; et al. Multivalent Calixarene-Based Liposomes as Platforms for Gene and Drug Delivery. Pharmaceutics 2021, 13, 1250. [Google Scholar] [CrossRef]
- Keck, C.M.; Specht, D.; Brussler, J. Influence of lipid matrix composition on biopharmaceutical properties of lipid nanoparticles. J. Control. Release 2021, 338, 149–163. [Google Scholar] [CrossRef]
- Anantaworasakul, P.; Anuchapreeda, S.; Yotsawimonwat, S.; Naksuriya, O.; Lekawanvijit, S.; Tovanabutra, N.; Anantaworasakul, P.; Wattanasri, W.; Buranapreecha, N.; Ampasavate, C. Nanomaterial Lipid-Based Carrier for Non-Invasive Capsaicin Delivery; Manufacturing Scale-Up and Human Irritation Assessment. Molecules 2020, 25, 5575. [Google Scholar] [CrossRef]
- Shahraeini, S.S.; Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Abootorabi, S.; Dehghanpoor, M.; Rostamkalaei, S.S.; Nokhodchi, A. Atorvastatin Solid Lipid Nanoparticles as a Promising Approach for Dermal Delivery and an Anti-inflammatory Agent. AAPS PharmSciTech 2020, 21, 263. [Google Scholar] [CrossRef]
- Rostamkalaei, S.S.; Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Nokhodchi, A. Topical gel of Metformin solid lipid nanoparticles: A hopeful promise as a dermal delivery system. Colloids Surf. B Biointerfaces 2019, 175, 150–157. [Google Scholar] [CrossRef]
- Essaghraoui, A.; Belfkira, A.; Hamdaoui, B.; Nunes, C.; Lima, S.A.C.; Reis, S. Improved Dermal Delivery of Cyclosporine A Loaded in Solid Lipid Nanoparticles. Nanomaterials 2019, 9, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza Guedes, L.; Martinez, R.M.; Bou-Chacra, N.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. An Overview on Topical Administration of Carotenoids and Coenzyme Q10 Loaded in Lipid Nanoparticles. Antioxidants 2021, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.; Van, M.; Thi, H.; Thanh, C.; Ngoc, B.; Van, B.; Thien, G.; Van, B.; Nguyen, C. Development of ibuprofen-loaded solid lipid nanoparticle-based hydrogels for enhanced in vitro dermal permeation and in vivo topical anti-inflammatory activity. J. Drug Deliv. Sci. Technol. 2020, 57, 101758. [Google Scholar] [CrossRef]
- Amasya, G.; Ozturk, C.; Aksu, B.; Tarimci, N. QbD based formulation optimization of semi-solid lipid nanoparticles as nano-cosmeceuticals. J. Drug Deliv. Sci. Technol. 2021, 66, 102737. [Google Scholar] [CrossRef]
- Boskabadi, M.; Saeedi, M.; Akbari, J.; Morteza-Semnani, K.; Hassan-Hashemi, S.; Babaei, A. Topical Gel of Vitamin A Solid Lipid Nanoparticles: A Hopeful Promise as a Dermal Delivery System. Adv. Pharm. Bull. 2021, 11, 663–674. [Google Scholar] [CrossRef]
- Qiu, T.; Gao, S.; Liang, Z.; Wang, D.G.; Tabassum, H.; Zhong, R.; Zou, R. Pristine Hollow Metal-Organic Frameworks: Design, Synthesis and Application. Angew. Chem. Int. Ed. Engl. 2021, 60, 17314–17336. [Google Scholar] [CrossRef]
- Morris, R.E.; Wheatley, P.S. Gas storage in nanoporous materials. Angew. Chem. Int. Ed. Engl. 2008, 47, 4966–4981. [Google Scholar] [CrossRef]
- Dhurjad, P.; Dhalaram, C.S.; Ali, N.; Kumari, N.; Sonti, R. Metal-organic frameworks in chiral separation of pharmaceuticals. Chirality 2022, 34, 1419–1436. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhao, S.N.; Zang, S.Q.; Li, J. Functional metal-organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef]
- Haider, J.; Shahzadi, A.; Akbar, M.U.; Hafeez, I.; Shahzadi, I.; Khalid, A.; Ashfaq, A.; Ahmad, S.O.A.; Dilpazir, S.; Imran, M.; et al. A review of synthesis, fabrication, and emerging biomedical applications of metal-organic frameworks. Biomater. Adv. 2022, 140, 213049. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qu, X.; Liu, C.; Xu, Q.; Tu, K. Metal-Organic Frameworks and Their Composites Towards Biomedical Applications. Front. Mol. Biosci. 2021, 8, 805228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, T.; Liang, K.; Chandrawati, R. Metal-organic frameworks for therapeutic gas delivery. Adv. Drug Deliv. Rev. 2021, 171, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; He, Q.; Yan, J.; Xiong, H.; Wen, N.; Cai, S.; Peng, D.; Liu, Y.; Liu, Z. Metal-organic frameworks for virus detection. Biosens. Bioelectron. 2020, 169, 112604. [Google Scholar] [CrossRef]
- Mao, H.; Yu, L.; Tu, M.; Wang, S.; Zhao, J.; Zhang, H.; Cao, Y. Recent Advances on the Metal-Organic Frameworks-Based Biosensing Methods for Cancer Biomarkers Detection. Crit. Rev. Anal. Chem. 2022, 1–17. [Google Scholar] [CrossRef]
- Muldoon, P.F.; Collet, G.; Eliseeva, S.V.; Luo, T.Y.; Petoud, S.; Rosi, N.L. Ship-in-a-Bottle Preparation of Long Wavelength Molecular Antennae in Lanthanide Metal-Organic Frameworks for Biological Imaging. J. Am. Chem Soc. 2020, 142, 8776–8781. [Google Scholar] [CrossRef]
- Tamames-Tabar, C.; Imbuluzqueta, E.; Guillou, N.; Serre, C.B.S.R.M.; Elkaïm, E.C.P.H.; Blanco-Prieto, M.J. A Zn azelate MOF: Combining antibacterial effect. CrystEngComm 2015, 17, 456–462. [Google Scholar] [CrossRef]
- Quaresma, S.; Andre, V.; Antunes, A.M.M.; Vilela, S.M.F.; Amariei, G.; Arenas-Vivo, A.; Rosal, R.; Horcajada, P.; Duarte, M.T. Novel Antibacterial Azelaic Acid BioMOFs. Cryst. Growth Des. 2020, 20, 370–382. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Liu, X.; Yin, Y.; Ma, Y.-H.; Gao, Y.; Wang, Y.; Lu, Z.; Song, Y. Gallium–Carbenicillin Framework Coated Defect-Rich Hollow TiO2 as a Photocatalyzed Oxidative Stress Amplifier against Complex Infections. Adv. Funct. Mater. 2020, 30, 2004861. [Google Scholar] [CrossRef]
- Duan, F.; Feng, X.; Jin, Y.; Liu, D.; Yang, X.; Zhou, G.; Liu, D.; Li, Z.; Liang, X.J.; Zhang, J. Metal-carbenicillin framework-based nanoantibiotics with enhanced penetration and highly efficient inhibition of MRSA. Biomaterials 2017, 144, 155–165. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, Y.; Huddleston, S.; Li, P.; Xiao, B.; Farha, O.K.; Ameer, G.A. Copper Metal-Organic Framework Nanoparticles Stabilized with Folic Acid Improve Wound Healing in Diabetes. ACS Nano 2018, 12, 1023–1032. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.; Ren, J.; Qu, X. A series of MOF/Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria. Biomaterials 2019, 208, 21–31. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Deng, Q.; Sang, Y.; Dong, K.; Ren, J.; Qu, X. Nature-Inspired Construction of MOF@COF Nanozyme with Active Sites in Tailored Microenvironment and Pseudopodia-Like Surface for Enhanced Bacterial Inhibition. Angew. Chem. Int. Ed. Engl. 2021, 60, 3469–3474. [Google Scholar] [CrossRef]
- Ren, X.; Yang, C.; Zhang, L.; Li, S.; Shi, S.; Wang, R.; Zhang, X.; Yue, T.; Sun, J.; Wang, J. Copper metal-organic frameworks loaded on chitosan film for the efficient inhibition of bacteria and local infection therapy. Nanoscale 2019, 11, 11830–11838. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, S.; Yi, J.; Zhang, H.; Ameer, G.A. A Cooperative Copper Metal-Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Zhu, G.; Zhu, P.; Ma, J.; Chen, W.; Liu, Z.; Kong, T. Omniphobic ZIF-8@Hydrogel Membrane by Microfluidic-Emulsion-Templating Method for Wound Healing. Adv. Funct. Mater. 2020, 30, 1909389. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Wu, X.; Wang, G.; Gu, G.; Wang, F.; Ren, J.; Zhang, H.; Zhao, Y. Microfluidic Electrospray Niacin Metal-Organic Frameworks Encapsulated Microcapsules for Wound Healing. Research 2019, 2019, 6175398. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Li, Y.; Liu, X.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K.W.K.; Li, C.; Cui, Z.; Liang, Y.; et al. Rapid bacteria trapping and killing of metal-organic frameworks strengthened photo-responsive hydrogel for rapid tissue repair of bacterial infected wounds. Chem. Eng. J. 2020, 396, 125194. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Wang, D.; Zheng, Y.; Li, Y.; Meng, W.; Zhang, X.; Du, F.; Lee, S. Ag@MOF-loaded chitosan nanoparticle and polyvinyl alcohol/sodium alginate/chitosan bilayer dressing for wound healing applications. Int. J. Biol. Macromol. 2021, 175, 481–494. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, R.; Hu, J. Engineering Metal-Organic Frameworks (MOFs) for Controlled Delivery of Physiological Gaseous Transmitters. Nanomaterials 2020, 10, 1134. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, e1801210. [Google Scholar] [CrossRef]
- Pinto, R.V.; Wang, S.; Tavares, S.R.; Pires, J.; Antunes, F.; Vimont, A.; Clet, G.; Daturi, M.; Maurin, G.; Serre, C.; et al. Tuning Cellular Biological Functions Through the Controlled Release of NO from a Porous Ti-MOF. Angew. Chem. Int. Ed. Engl. 2020, 59, 5135–5143. [Google Scholar] [CrossRef]
- Pinto, R.V.; Antunes, F.; Pires, J.; Graca, V.; Brandao, P.; Pinto, M.L. Vitamin B3 metal-organic frameworks as potential delivery vehicles for therapeutic nitric oxide. Acta Biomater. 2017, 51, 66–74. [Google Scholar] [CrossRef]
- Marquez, A.G.; Hidalgo, T.; Lana, H.; Cunha, D.; Blanco-Prieto, M.J.; Alvarez-Lorenzo, C.; Boissiere, C.; Sanchez, C.; Serre, C.; Horcajada, P. Biocompatible polymer-metal-organic framework composite patches for cutaneous administration of cosmetic molecules. J. Mater. Chem. B 2016, 4, 7031–7040. [Google Scholar] [CrossRef]
- Osorio-Toribio, G.; Velasquez-Hernandez, M.J.; Mileo, P.G.M.; Zarate, J.A.; Aguila-Rosas, J.; Leyva-Gomez, G.; Sanchez-Sanchez, R.; Magana, J.J.; Perez-Diaz, M.A.; Lazaro, I.A.; et al. Controlled Transdermal Release of Antioxidant Ferulate by a Porous Sc(III) MOF. iScience 2020, 23, 101156. [Google Scholar] [CrossRef]
- Taherzade, S.D.; Rojas, S.; Soleimannejad, J.; Horcajada, P. Combined Cutaneous Therapy Using Biocompatible Metal-Organic Frameworks. Nanomaterials 2020, 10, 2296. [Google Scholar] [CrossRef]
- Li, J.; Lv, F.; Li, J.; Li, Y.; Gao, J.; Luo, J.; Xue, F.; Ke, Q.; Xu, H. Cobalt-based metal–organic framework as a dual cooperative controllable release system for accelerating diabetic wound healing. Nano Res. 2020, 13, 2268–2279. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Allan, P.K.; Renouf, C.L.; Duncan, M.J.; Wheatley, P.S.; Warrender, S.J.; Dawson, D.; Ashbrook, S.E.; Gil, B.; Marszalek, B.; et al. Multirate delivery of multiple therapeutic agents from metal-organic frameworks. APL Mater. 2014, 2, 124108. [Google Scholar] [CrossRef]



| Type of Nanoparticle | Formulation | Aims | Reference |
|---|---|---|---|
| Inorganic | SiO/peptide | Reduce the inflammatory response liberating the natural compound from the nanoparticle mesoporous. | [73] |
| Inorganic | ZnO | Prevent microbial infection and decrease the ROS level on the skin. | [74] |
| Inorganic | Nanocomposite of zinc and silver nanocomposite | Promotes the healing of skin wounds | [75] |
| Inorganic | Super paramagnetic Iron Oxide/polyethelyimine | Skin penetration via follicular pathways | [76] |
| Polymeric | Polycaprolactone/gum arabic/ZnO nanocomposite | Promote the regeneration of skin tissue and treat difficult healing skin wounds. | [77] |
| Polymeric | Dexamethasone loaded dentritic nanoparticle | Reduce the inflammatory response of atopic dermatitis. | [78] |
| Polymeric | SIlibilin encapsulated in gellan gum | Platform for skin delivery. | [79] |
| Lipidic | Raloxifene-loaded cubosomes | Transdermal delivery | [80] |
| Lipidic | Cyproterone encapsulated into nanostructure lipid carriers | Promote penetration via follicular appendages. | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, J.; Carmona, T.; Zacconi, F.C.; Venegas-Yazigi, D.; Cabello-Verrugio, C.; Il Choi, W.; Vilos, C. The Human Dermis as a Target of Nanoparticles for Treating Skin Conditions. Pharmaceutics 2023, 15, 10. https://doi.org/10.3390/pharmaceutics15010010
Salazar J, Carmona T, Zacconi FC, Venegas-Yazigi D, Cabello-Verrugio C, Il Choi W, Vilos C. The Human Dermis as a Target of Nanoparticles for Treating Skin Conditions. Pharmaceutics. 2023; 15(1):10. https://doi.org/10.3390/pharmaceutics15010010
Chicago/Turabian StyleSalazar, Javier, Thais Carmona, Flavia C. Zacconi, Diego Venegas-Yazigi, Claudio Cabello-Verrugio, Won Il Choi, and Cristian Vilos. 2023. "The Human Dermis as a Target of Nanoparticles for Treating Skin Conditions" Pharmaceutics 15, no. 1: 10. https://doi.org/10.3390/pharmaceutics15010010