Towards Highly Loaded and Finely Dispersed CuO Catalysts via ADP: Effect of the Alumina Support
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Properties of the Alumina Supports
2.2. Physicochemical Properties of the Copper-Loaded Alumina Catalysts
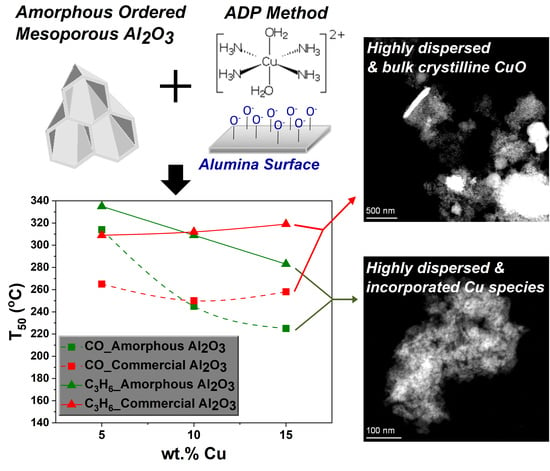
2.3. Catalytic Activity of CuO/Alumina
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.1.1. Synthesis of Alumina Supports
- Ethanol-based method—Al(1)
- Water-based method—Al(2)
- Commercially Available Al2O3—Al(3)
- Flame Spray Pyrolysis method—Al(4)
4.1.2. CuO Loading Using the ADP Method
4.2. Physicochemical Characterization
4.3. Catalytic Activity Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Twigg, M.V. Catalytic control of emissions from cars. Catal. Today 2011, 163, 33–41. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Federal Register Vol.79 No.81. 2014. Available online: https://www.govinfo.gov/content/pkg/FR-2014-04-28/pdf/2014-06954.pdf (accessed on 28 April 2014).
- Environmental Protection Agency (EPA). Amendments Related to: Tier 3 Motor Vehicle Emission and Fuel Standards, Environmental Protection Agency (EPA). 2016. Available online: https://www.epa.gov/regulations-emissions-vehicles-and-engines/final-rule-amendments-related-tier-3-motor-vehicle (accessed on 22 April 2016).
- Wilson, N.; Horrocks, J. Lessons from the removal of lead from gasoline for controlling other environmental pollutants: A case study from New Zealand. Environ. Health 2008, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yao, X.; Lu, Y.; Sun, C.; Tang, C.; Gao, F.; Dong, L. Effect of precursors on the structure and activity of CuO-CoOx/γ-Al2O3 catalysts for NO reduction by CO. J. Colloid Interf. Sci. 2018, 509, 334–345. [Google Scholar] [CrossRef]
- Peneau, V.; Shaw, G.; Armstrong, R.D.; Jenkins, R.L.; Dimitratos, N.; Taylor, S.H.; Zanthoff, H.W.; Peitz, S.; Stochniol, G.; Hutchings, G.J. The partial oxidation of propane under mild aqueous conditions with H2O2 and ZSM-5 catalysts. Catal. Sci. Technol. 2016, 6, 7521–7531. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Wang, J.; Chai, W.; Zhu, J.; Men, Y. Enhanced soot oxidation activity over CuO/CeO2 mesoporous nanosheets. Catal. Sci. Technol. 2019, 9, 1699–1709. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Tang, W.; He, J.; Miao, R.; Lin, H.-J.; Song, W.; Wang, S.; Gao, P.-X.; Suib, S.L. Copper manganese oxide enhanced nanoarray-based monolithic catalysts for hydrocarbon oxidation. J. Mater. Chem. A 2018, 6, 19047–19057. [Google Scholar] [CrossRef]
- Patel, A.; Shukla, P.; Chen, J.; Rufford, T.E.; Wang, S.; Rudolph, V.; Zhu, Z. Structural sensitivity of mesoporous alumina for copper catalyst loading used for NO reduction in presence of CO. Chem. Eng. Res. Des. 2015, 101, 27–43. [Google Scholar] [CrossRef]
- Patel, A.; Rufford, T.; Rudolph, V.; Zhu, Z. Selective catalytic reduction of NO by CO over CuO supported on SBA-15: Effect of CuO loading on the activity of catalysts. Catal. Today 2011, 166, 188–193. [Google Scholar] [CrossRef]
- Lyu, X.; Wang, P.; Zhao, H.; Zhang, Y.; Meng, Z.; Lei, L. Migration mechanism on Cu species in CHA-type catalyst for the selective catalytic reduction of NOx during hydrothermal treatment. J. Environ. Chem. Eng. 2021, 9, 106413–106423. [Google Scholar] [CrossRef]
- Yoshida, H.; Hirakawa, T.; Oyama, H.; Nakashima, R.; Hinokuma, S.; Machida, M. Effect of Thermal Aging on Local Structure and Three-Way Catalysis of Cu/Al2O3. J. Phys. Chem. C 2019, 123, 10469–10476. [Google Scholar] [CrossRef]
- Yoshida, H.; Yamashita, N.; Ijichi, S.; Okabe, Y.; Misumi, S.; Hinokuma, S.; Machida, M. A Thermally Stable Cr–Cu Nanostructure Embedded in the CeO2 Surface as a Substitute for Platinum-Group Metal Catalysts. ACS Catal. 2015, 5, 6738–6747. [Google Scholar] [CrossRef]
- Renuka, N.; Shijina, A.; Praveen, A.; Aniz, C. Redox properties and catalytic activity of CuO/γ-Al2O3 meso phase. J. Colloid Interf. Sci. 2014, 434, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.-I.; Maeshima, H.; Yoshida, H.; Satsuma, A.; Hattori, T. Spectroscopic characterisation of Cu–Al2O3 catalysts for selective catalytic reduction of NO with propene. Phys. Chem. Chem. Phys. 2000, 2, 2435–2439. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Maeshima, H.; Satsuma, A.; Hattori, T. Transition metal-aluminate catalysts for NO reduction by C3H6. Appl. Catal. B Environ. 1998, 18, 163–170. [Google Scholar] [CrossRef]
- Patel, A.; Shukla, P.; Pan, G.T.; Chong, S.; Rudolph, V.; Zhu, Z. Influence of copper loading on mesoporous alumina for catalytic NO reduction in the presence of CO. J. Environ. Chem. Eng. 2017, 5, 2350–2361. [Google Scholar] [CrossRef]
- Prieto, G.; Zecevic, J.; Friedrich, H.; De Jong, K.P.; De Jongh, P.E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 2012, 12, 34–39. [Google Scholar] [CrossRef]
- Xin, Q.; Glisenti, A.; Philippopoulos, C.; Poulakis, E.; Mertens, M.; Nyalosaso, J.L.; Meynen, V.; Cool, P. Comparison between a Water-Based and a Solvent-Based Impregnation Method towards Dispersed CuO/SBA-15 Catalysts: Texture, Structure and Catalytic Performance in Automotive Exhaust Gas Abatement. Catalysts 2016, 6, 164. [Google Scholar] [CrossRef] [Green Version]
- Xin, Q.; Papavasiliou, A.; Boukos, N.; Glisenti, A.; Li, J.P.H.; Yang, Y.; Philippopoulos, C.J.; Poulakis, E.; Katsaros, F.K.; Meynen, V.; et al. Preparation of CuO/SBA-15 catalyst by the modified ammonia driven deposition precipitation method with a high thermal stability and an efficient automotive CO and hydrocarbons conversion. Appl. Catal. B Environ. 2018, 223, 103–115. [Google Scholar] [CrossRef]
- Jiao, L.; Regalbuto, J.R. The synthesis of highly dispersed noble and base metals on silica via strong electrostatic adsorption: I. Amorphous silica. J. Catal. 2008, 260, 329–341. [Google Scholar] [CrossRef]
- Ge, C.; Liu, L.; Liu, Z.; Yao, X.; Cao, Y.; Tang, C.; Gao, F.; Dong, L. Improving the dispersion of CeO2 on γ-Al2O3 to enhance the catalytic performances of CuO/CeO2/γ-Al2O3 catalysts for NO removal by CO. Catal. Commun. 2014, 51, 95–99. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, S.; Zhang, C.; O’Hare, D.; Wang, Q. Synthesis of Cu0.5Mg1.5Mn0.5Al0.5Ox mixed oxide from layered double hydroxide precursor as highly efficient catalyst for low-temperature selective catalytic reduction of NOx with NH3. J. Colloid Interf. Sci. 2018, 526, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wan, Z.; Chen, W.; Zhu, M.; Zhang, D. Synthesis of mesoporous alumina with tunable structural properties. Microporous Mesoporous Mater. 2015, 217, 12–20. [Google Scholar] [CrossRef]
- Yuan, Q.; Yin, A.-X.; Luo, C.; Sun, L.-D.; Zhang, Y.-W.; Duan, W.-T.; Liu, A.H.-C.; Yan, C.-H. Facile Synthesis for Ordered Mesoporous γ-Aluminas with High Thermal Stability. J. Am. Chem. Soc. 2008, 130, 3465–3472. [Google Scholar] [CrossRef]
- Cai, W.; Yu, J.; Anand, C.; Vinu, A.; Jaroniec, M. Facile Synthesis of Ordered Mesoporous Alumina and Alumina-Supported Metal Oxides with Tailored Adsorption and Framework Properties. Chem. Mater. 2011, 23, 1147–1157. [Google Scholar] [CrossRef]
- Wu, W.; Wan, Z.; Zhu, M.; Zhang, D. A facile route to aqueous phase synthesis of mesoporous alumina with controllable structural properties. Microporous Mesoporous Mater. 2016, 223, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Duan, H.-H.; Li, L.-L.; Li, Z.-X.; Duan, W.-T.; Zhang, L.-S.; Song, W.-G.; Yan, C.-H. Homogeneously Dispersed Ceria Nanocatalyst Stabilized with Ordered Mesoporous Alumina. Adv. Mater. 2010, 22, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Hafshejani, L.D.; Tangsir, S.; Koponen, H.; Riikonen, J.; Karhunen, T.; Tapper, U.; Lehto, V.-P.; Moazed, H.; Naseri, A.A.; Hooshmand, A.; et al. Synthesis and characterization of Al2O3 nanoparticles by flame spray pyrolysis (FSP)—Role of Fe ions in the precursor. Powder Technol. 2016, 298, 42–49. [Google Scholar] [CrossRef]
- Chen, L.; Horiuchi, T.; Osaki, T.; Mori, T. Catalytic selective reduction of NO with propylene over Cu-Al2O3 catalysts: Influence of catalyst preparation method. Appl. Catal. B Environ. 1999, 23, 259–269. [Google Scholar] [CrossRef]
- Guo, X.; Yin, A.; Dai, W.-L.; Fan, K. One Pot Synthesis of Ultra-High Copper Contented Cu/SBA-15 Material as Excellent Catalyst in the Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. Catal. Lett. 2009, 132, 22–27. [Google Scholar] [CrossRef]
- Lopez-Suarez, F.E.; Bueno-López, A.; Illán-Gómez, M. Cu/Al2O3 catalysts for soot oxidation: Copper loading effect. Appl. Catal. B Environ. 2008, 84, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Papavasiliou, A.; Van Everbroeck, T.; Blonda, C.; Oliani, B.; Sakellis, E.; Cool, P.; Canu, P.; Katsaros, F.K. Mesoporous CuO/TiO2 catalysts prepared by the ammonia driven deposition precipitation method for CO preferential oxidation: Effect of metal loading. Fuel 2022, 311, 122491. [Google Scholar] [CrossRef]
- Strohmeier, B.R.; Levden, D.E.; Field, R.S.; Hercules, D.M. Surface spectroscopic characterization of Cu/Al2O3 catalysts. J. Catal. 1985, 94, 514–530. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, B.; Wang, K.-K.; Ling, G.-P.; Cai, J.; Song, C.-L.; Han, G.-R. Study of Raman spectra for γ-Al2O3 models by using first-principles method. Solid State Commun. 2014, 178, 16–22. [Google Scholar] [CrossRef]
- Le Nestour, A.; Gaudon, M.; Villeneuve, G.; Daturi, M.; Andriessen, A.R.; Demourgues, A. Defects in Divided Zinc−Copper Aluminate Spinels: Structural Features and Optical Absorption Properties. Inorg. Chem. 2007, 46, 4067–4078. [Google Scholar] [CrossRef]
- Ertl, G.; Hierl, R.; Knözinger, H.; Thiele, N.; Urbach, H. XPS study of copper aluminate catalysts. Appl. Surf. Sci. 1980, 5, 49–64. [Google Scholar] [CrossRef]
- Tikhov, S.; Sadykov, V.; Kryukova, G.; Paukshtis, E.; Popovskii, V.; Starostina, T.; Kharlamov, G.; Anufrienko, V.; Poluboyarov, V.; Razdobarov, V. Microstructural and spectroscopic investigations of the supported copperalumina oxide system: Nature of aging in oxidizing reaction media. J. Catal. 1992, 134, 506–524. [Google Scholar] [CrossRef]
- Severino, F.; Brito, J.L.; Laine, J.; Fierro, J.; Agudo, A. Nature of Copper Active Sites in the Carbon Monoxide Oxidation on CuAl2O4 and CuCr2O4 Spinel Type Catalysts. J. Catal. 1998, 177, 82–95. [Google Scholar] [CrossRef]
- Papavasiliou, A.; Deze, E.; Papageorgiou, S.; Sideratou, Z.; Boukos, N.; Poulakis, E.; Philippopoulos, C.; Glisenti, A.; Van Everbroeck, T.; Cool, P.; et al. A hyperbranched polymer synthetic strategy for the efficient fixation of metal species within nanoporous structures: Application in automotive catalysis. Chem. Eng. J. 2021, 421, 129496. [Google Scholar] [CrossRef]
- Jabłońska, M.; Beale, A.M.; Nocuń, M.; Palkovits, R. Ag-Cu based catalysts for the selective ammonia oxidation into nitrogen and water vapour. Appl. Catal. B Environ. 2018, 232, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Amano, F.; Suzuki, S.; Yamamoto, T.; Tanaka, T. One-electron reducibility of isolated copper oxide on alumina for selective NO–CO reaction. Appl. Catal. B Environ. 2006, 64, 282–289. [Google Scholar] [CrossRef]
- Zhou, R.-X.; Yu, T.-M.; Jiang, X.-Y.; Chen, F.; Zheng, X.-M. Temperature-programmed reduction and temperature-programmed desorption studies of CuO/ZrO2 catalysts. Appl. Surf. Sci. 1999, 148, 263–270. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Liu, A.; Yu, Y.; Ji, J.; Guo, K.; Wan, H.; Tang, C.; Dong, L. Unravelling the structure sensitivity of CuO/SiO2 catalysts in the NO + CO reaction. Catal. Sci. Technol. 2020, 10, 3848–3856. [Google Scholar] [CrossRef]
- Urrutxua, M.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Evaluation of Cu/SAPO-34 Catalysts Prepared by Solid-State and Liquid Ion-Exchange Methods for NOx Removal by NH3-SCR. ACS Omega 2019, 4, 14699–14713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, R.M.; Freeman, J.J.; Lytle, F.W. Characterization of CuAl2O3 catalysts. J. Catal. 1978, 55, 10–28. [Google Scholar] [CrossRef]
- Ham, H.; Kim, J.; Cho, S.J.; Choi, J.-H.; Moon, D.J.; Bae, J.W. Enhanced Stability of Spatially Confined Copper Nanoparticles in an Ordered Mesoporous Alumina for Dimethyl Ether Synthesis from Syngas. ACS Catal. 2016, 6, 5629–5640. [Google Scholar] [CrossRef]
- Wolberg, A.; Roth, J. Copper oxide supported on alumina III. X-ray K-absorption edge studies of the Cu2+ species. J. Catal. 1969, 15, 250–255. [Google Scholar] [CrossRef]
- Fu, L.; Li, X.; Liu, M.; Yang, H. Insights into the nature of Cu doping in amorphous mesoporous alumina. J. Mater. Chem. A 2013, 1, 14592–14605. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, H.; Wang, S.; Lu, G. Preparation and Characterization of Copper Catalysts Supported on Mesoporous Al2O3Nanofibers for N2O Reduction to N2. Catal. Lett. 2003, 91, 73–81. [Google Scholar] [CrossRef]
- Gang, L.; van Grondelle, J.; Anderson, B.G.; van Santen, R.A. Selective Low Temperature NH3 Oxidation to N2 on Copper-Based Catalysts. J. Catal. 1999, 186, 100–109. [Google Scholar] [CrossRef]
- Van Everbroeck, T.; Ciocarlan, R.-G.; Van Hoey, W.; Mertens, M.; Cool, P. Copper-Containing Mixed Metal Oxides (Al, Fe, Mn) for Application in Three-Way Catalysis. Catalysts 2020, 10, 1344. [Google Scholar] [CrossRef]
- Qian, J.; Hou, X.; Qin, Z.; Li, B.; Tong, Z.; Dong, L.; Dong, L. Enhanced catalytic properties of Cu-based composites for NOx reduction with coexistence and intergrowth effect. Fuel 2018, 234, 296–304. [Google Scholar] [CrossRef]
- Hirakawa, T.; Shimokawa, Y.; Tokuzumi, W.; Sato, T.; Tsushida, M.; Yoshida, H.; Ohyama, J.; Machida, M. Multicomponent 3d Transition-Metal Nanoparticles as Catalysts Free of Pd, Pt, or Rh for Automotive Three-Way Catalytic Converters. ACS Appl. Nano Mater. 2020, 3, 9097–9107. [Google Scholar] [CrossRef]
- Norouzzadeh, P.; Mabhouti, K.; Golzan, M.; Naderali, R. Investigation of structural, morphological and optical characteristics of Mn substituted Al-doped ZnO NPs: A Urbach energy and Kramers-Kronig study. Optik 2020, 204, 164227. [Google Scholar] [CrossRef]













| Sample | Method for Al2O3 | Cu wt% |
|---|---|---|
| Al(1) | Ethanol | - |
| 5Cu Al(1) | Ethanol | 5 |
| 10Cu Al(1) | Ethanol | 10 |
| 15Cu Al(1) | Ethanol | 15 |
| Al(2) | Water | - |
| 5Cu Al(2) | Water | 5 |
| 10Cu Al(2) | Water | 10 |
| 15Cu Al(2) | Water | 15 |
| Al(3) | Commercial | - |
| 5Cu Al(3) | Commercial | 5 |
| 10Cu Al(3) | Commercial | 10 |
| 15Cu Al(3) | Commercial | 15 |
| Al(4) | FSP | - |
| 5Cu Al(4) | FSP | 5 |
| 10Cu Al(4) | FSP | 10 |
| 15Cu Al(4) | FSP | 15 |
| Sample | BET SSA m2 g−1 | TPV cc g−1 | APD nm | NH3 (m/z = 16) µmol g−1 |
|---|---|---|---|---|
| Al(1) | 286 | 0.45 | 6.3 | 66 |
| Al(2) | 324 | 1.18 | 18 | 94 |
| Al(3) | 127 | 0.42 | 30 | 32 |
| Al(4) | 112 | 0.21 | 3 | 26 |
| Sample | BET SSA m2 g−1 | TPV cc g−1 | APD nm | Mean Crystal Size nm |
|---|---|---|---|---|
| 5Cu Al(1) | 256 | 0.27 | 4.3 | - |
| 10Cu Al(1) | 250 | 0.24 | 3.9 | - |
| 15Cu Al(1) | 241 | 0.25 | 4.2 | - |
| 5Cu Al(2) | 288 | 0.65 | 8 | - |
| 10Cu Al(2) | 258 | 0.75 | 10 | - |
| 15Cu Al(2) | 265 | 0.80 | 13 | 21 |
| 5Cu Al(3) | 120 | 0.39 | 30 | - |
| 10Cu Al(3) | 105 | 0.42 | 31 | 20 |
| 15Cu Al(3) | 107 | 0.44 | 32 | 20 |
| 5Cu Al(4) | 100 | 0.27 | 10 | - |
| 10Cu Al(4) | 96 | 0.34 | 18 | 19 |
| 15Cu Al(4) | 79 | 0.26 | 13 | 18 |
| Sample | Cu 2p3/2 BE (eV) a | % Cu0 b | Al 2p BE (eV) a | Cu Surf. (wt%) c | Cu (wt%) d | Cu/Al Atomic Ratio e |
|---|---|---|---|---|---|---|
| 15 Cu Al(1) | 934.88 | 29 | 74.48 | 8.88 | 13.5 | 2.4 |
| 15 Cu Al(2) | 934.58 | 34 | 74.08 | 7.8 | 14.9 | 2 |
| 15 Cu Al(3) | 933.08 | 43 | 74.18 | 6.55 | 14.4 | 1.5 |
| 15 Cu Al(4) | 933.28 | 41 | 74.08 | 7.89 | 14.2 | 1.9 |
| Gas | CO | CH4 | C3H6 | C3H8 | CO2 | H2O | H2 | O2 | NO |
|---|---|---|---|---|---|---|---|---|---|
| Vol. % | 0.7 | 0.0225 | 0.0450 | 0.0225 | 15 | 10 | 0.233 | 0.777 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Everbroeck, T.; Papavasiliou, A.; Ciocarlan, R.-G.; Poulakis, E.; Philippopoulos, C.J.; Jardim, E.O.; Silvestre-Albero, J.; Sakellis, E.; Cool, P.; Katsaros, F.K. Towards Highly Loaded and Finely Dispersed CuO Catalysts via ADP: Effect of the Alumina Support. Catalysts 2022, 12, 628. https://doi.org/10.3390/catal12060628
Van Everbroeck T, Papavasiliou A, Ciocarlan R-G, Poulakis E, Philippopoulos CJ, Jardim EO, Silvestre-Albero J, Sakellis E, Cool P, Katsaros FK. Towards Highly Loaded and Finely Dispersed CuO Catalysts via ADP: Effect of the Alumina Support. Catalysts. 2022; 12(6):628. https://doi.org/10.3390/catal12060628
Chicago/Turabian StyleVan Everbroeck, Tim, Aggeliki Papavasiliou, Radu-George Ciocarlan, Evangelos Poulakis, Constantine J. Philippopoulos, Erika O. Jardim, Joaquin Silvestre-Albero, Elias Sakellis, Pegie Cool, and Fotios K. Katsaros. 2022. "Towards Highly Loaded and Finely Dispersed CuO Catalysts via ADP: Effect of the Alumina Support" Catalysts 12, no. 6: 628. https://doi.org/10.3390/catal12060628