Does the Application of Additional Hydrophobic Resin to Universal Adhesives Increase Bonding Longevity of Eroded Dentin?
Abstract
:1. Introduction
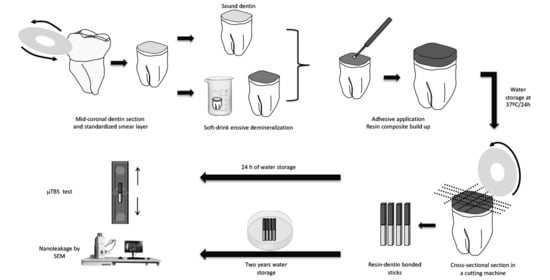
2. Materials and Methods
2.1. Tooth Selection and Preparation
2.2. Experimental Design
2.3. Sample Size Calculation
2.4. pH Cycling Model
2.5. Restorative Procedures
2.6. Microtensile Bond Strength Test (μTBS)
2.7. Nanoleakage (NL)
2.8. Statistical Analysis
3. Results
3.1. Microtensile Bond Strength (μTBS)
3.2. Nanoleakage (NL)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmerli, B.; De Munck, J.; Lussi, A.; Lambrechts, P.; Van Meerbeek, B. Long-term bonding to eroded dentin requires superficial bur preparation. Clin. Oral Investig. 2012, 16, 1451–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, F.S.F.; Cardenas, A.M.; Ocampo, J.B.; Hass, V.; Bandeca, M.C.; Gomes, J.C.; Reis, A.; Loguercio, A.D. Bonding performance of universal adhesives to eroded dentin. J. Adhes. Dent. 2018, 20, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Forgerini, T.V.; Ribeiro, J.F.; Rocha, R.O.; Soares, F.Z.; Lenzi, T.L. Role of etching mode on bonding longevity of a universal adhesive to eroded dentin. J. Adhes. Dent. 2017, 19, 69–75. [Google Scholar] [CrossRef] [PubMed]
- de Rossi, G.R.C.; Ozcan, M.; Volpato, C.A.M. How to improve bond stability to eroded dentin: A comprehensive review. J. Adhes. Sci. Technol. 2021, 35, 1015–1034. [Google Scholar] [CrossRef]
- Prati, C.; Montebugnoli, L.; Suppa, P.; Valdre, G.; Mongiorgi, R. Permeability and morphology of dentin after erosion induced by acidic drinks. J. Periodontol. 2003, 74, 428–436. [Google Scholar] [CrossRef]
- Sano, H.; Shono, T.; Takatsu, T.; Hosoda, H. Microporous dentin zone beneath resin-impregnated layer. Oper. Dent. 1994, 19, 59–64. [Google Scholar]
- Zarella, B.L.; Cardoso, C.A.; Pela, V.T.; Kato, M.T.; Tjaderhane, L.; Buzalaf, M.A. The role of matrix metalloproteinases and cysteine-cathepsins on the progression of dentine erosion. Arch. Oral Biol. 2015, 60, 1340–1345. [Google Scholar] [CrossRef]
- Tjaderhane, L.; Buzalaf, M.A.; Carrilho, M.; Chaussain, C. Matrix metalloproteinases and other matrix proteinases in relation to cariology: The era of ‘dentin degradomics’. Caries Res. 2015, 49, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, T.S.; Colon, P.; Ganss, C.; Huysmans, M.C.; Lussi, A.; Schlueter, N.; Schmalz, G.; Shellis, P.R.; Bjorg Tveit, A.; Wiegand, A. Consensus Report of the European Federation of Conservative Dentistry: Erosive tooth wear diagnosis and management. Swiss Dent. J. 2016, 126, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lussi, A. Assessment and management of dental erosion. Dent. Clin. N. Am. 2010, 54, 565–578. [Google Scholar] [CrossRef]
- Siqueira, F.; Cardenas, A.; Gomes, G.M.; Chibinski, A.C.; Gomes, O.; Bandeca, M.C.; Loguercio, A.D.; Gomes, J.C. Three-year effects of deproteinization on the in vitro durability of resin/dentin-eroded interfaces. Oper. Dent. 2018, 43, 60–70. [Google Scholar] [CrossRef]
- de Siqueira, F.S.F.; Hilgemberg, B.; Araujo, L.C.R.; Hass, V.; Bandeca, M.C.; Gomes, J.C.; Reis, A.; Loguercio, A.D.; Cardenas, A.F.M. Improving bonding to eroded dentin by using collagen cross-linking agents: 2 years of water storage. Clin. Oral Investig. 2020, 24, 809–822. [Google Scholar] [CrossRef]
- Reis, A.; Carrilho, M.; Breschi, L.; Loguercio, A.D. Overview of clinical alternatives to minimize the degradation of the resin-dentin bonds. Oper. Dent. 2013, 38, E1–E25. [Google Scholar] [CrossRef] [Green Version]
- Ermis, R.B.; Ugurlu, M.; Ahmed, M.H.; Van Meerbeek, B. Universal Adhesives Benefit from an Extra Hydrophobic Adhesive Layer When Light Cured Beforehand. J. Adhes. Dent. 2019, 21, 179–188. [Google Scholar] [CrossRef]
- Munoz, M.A.; Sezinando, A.; Luque-Martinez, I.; Szesz, A.L.; Reis, A.; Loguercio, A.D.; Bombarda, N.H.; Perdigao, J. Influence of a hydrophobic resin coating on the bonding efficacy of three universal adhesives. J. Dent. 2014, 42, 595–602. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Yao, C.; Van Landuyt, K.; Peumans, M.; Van Meerbeek, B. Extra Bonding Layer Compensates Universal Adhesive’s Thin Film Thickness. J. Adhes. Dent. 2020, 22, 483–501. [Google Scholar] [CrossRef]
- Sezinando, A.; Luque-Martinez, I.; Munoz, M.A.; Reis, A.; Loguercio, A.D.; Perdigao, J. Influence of a hydrophobic resin coating on the immediate and 6-month dentin bonding of three universal adhesives. Dent. Mater. 2015, 31, e236–e246. [Google Scholar] [CrossRef]
- Perdigao, J.; Ceballos, L.; Giraldez, I.; Baracco, B.; Fuentes, M.V. Effect of a hydrophobic bonding resin on the 36-month performance of a universal adhesive-a randomized clinical trial. Clin. Oral Investig. 2020, 24, 765–776. [Google Scholar] [CrossRef]
- Albuquerque, M.; Pegoraro, M.; Mattei, G.; Reis, A.; Loguercio, A.D. Effect of double-application or the application of a hydrophobic layer for improved efficacy of one-step self-etch systems in enamel and dentin. Oper. Dent. 2008, 33, 564–570. [Google Scholar] [CrossRef]
- Reis, A.; Albuquerque, M.; Pegoraro, M.; Mattei, G.; Bauer, J.R.; Grande, R.H.; Klein-Junior, C.A.; Baumhardt-Neto, R.; Loguercio, A.D. Can the durability of one-step self-etch adhesives be improved by double application or by an extra layer of hydrophobic resin? J. Dent. 2008, 36, 309–315. [Google Scholar] [CrossRef]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef]
- Malacarne, J.; Carvalho, R.M.; de Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; Yiu, C.K.; Carrilho, M.R. Water sorption/solubility of dental adhesive resins. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef]
- Ito, S.; Hashimoto, M.; Wadgaonkar, B.; Svizero, N.; Carvalho, R.M.; Yiu, C.; Rueggeberg, F.A.; Foulger, S.; Saito, T.; Nishitani, Y.; et al. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 2005, 26, 6449–6459. [Google Scholar] [CrossRef]
- Tanaka, J.; Ishikawa, K.; Yatani, H.; Yamashita, A.; Suzuki, K. Correlation of dentin bond durability with water absorption of bonding layer. Dent. Mater. J. 1999, 18, 11–18. [Google Scholar] [CrossRef]
- Loguercio, A.D.; Reis, A. Application of a dental adhesive using the self-etch and etch-and-rinse approaches: An 18-month clinical evaluation. J. Am. Dent. Assoc. 2008, 139, 53–61. [Google Scholar] [CrossRef]
- Perdigao, J.; Munoz, M.A.; Sezinando, A.; Luque-Martinez, I.V.; Staichak, R.; Reis, A.; Loguercio, A.D. Immediate adhesive properties to dentin and enamel of a universal adhesive associated with a hydrophobic resin coat. Oper. Dent. 2014, 39, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.H.; De Munck, J.; Van Landuyt, K.; Peumans, M.; Yoshihara, K.; Van Meerbeek, B. Do universal adhesives benefit from an extra bonding layer? J. Adhes. Dent. 2019, 21, 117–132. [Google Scholar] [CrossRef]
- Belmar da Costa, M.; Delgado, A.H.S.; Pinheiro de Melo, T.; Amorim, T.; Mano Azul, A. Analysis of laboratory adhesion studies in eroded enamel and dentin: A scoping review. Biomater. Investig. Dent. 2021, 8, 24–38. [Google Scholar] [CrossRef]
- Munoz, M.A.; Luque, I.; Hass, V.; Reis, A.; Loguercio, A.D.; Bombarda, N.H. Immediate bonding properties of universal adhesives to dentine. J. Dent. 2013, 41, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Niu, L.N.; Xie, H.; Zhang, Z.Y.; Zhou, L.Q.; Jiao, K.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Bonding of universal adhesives to dentine--Old wine in new bottles? J. Dent. 2015, 43, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, S.; Breschi, L.; Ozcan, M.; Pfefferkorn, F.; Ferrari, M.; Van Meerbeek, B. Academy of Dental Materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (muTBS) approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhaes, A.C.; Levy, F.M.; Souza, B.M.; Cardoso, C.A.; Cassiano, L.P.; Pessan, J.P.; Buzalaf, M.A. Inhibition of tooth erosion by milk containing different fluoride concentrations: An in vitro study. J. Dent. 2014, 42, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Flury, S.; Koch, T.; Peutzfeldt, A.; Lussi, A.; Ganss, C. The effect of a tin-containing fluoride mouth rinse on the bond between resin composite and erosively demineralised dentin. Clin. Oral Investig. 2013, 17, 217–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdigao, J.; Geraldeli, S.; Carmo, A.R.; Dutra, H.R. In vivo influence of residual moisture on microtensile bond strengths of one-bottle adhesives. J. Esthet. Restor. Dent. 2002, 14, 31–38. [Google Scholar] [CrossRef]
- Reis, A.; Grande, R.H.; Oliveira, G.M.; Lopes, G.C.; Loguercio, A.D. A 2-year evaluation of moisture on microtensile bond strength and nanoleakage. Dent. Mater. 2007, 23, 862–870. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H.; Suh, B.I.; Carvalho, R.M.; Itthagarun, A. Single-step adhesives are permeable membranes. J. Dent. 2002, 30, 371–382. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Tay, F.R.; Doi, J.; Nishitani, Y.; Yamada, T.; Itou, K.; Carvalho, R.M.; Nakajima, M.; Pashley, D.H. Bonding of self-etch and total-etch adhesives to carious dentin. J. Dent. Res. 2002, 81, 556–560. [Google Scholar] [CrossRef]
- Hass, V.; Dobrovolski, M.; Zander-Grande, C.; Martins, G.C.; Gordillo, L.A.; Rodrigues Accorinte Mde, L.; Gomes, O.M.; Loguercio, A.D.; Reis, A. Correlation between degree of conversion, resin-dentin bond strength and nanoleakage of simplified etch-and-rinse adhesives. Dent. Mater. 2013, 29, 921–928. [Google Scholar] [CrossRef]
- Cuevas-Suarez, C.E.; da Rosa, W.L.O.; Lund, R.G.; da Silva, A.F.; Piva, E. Bonding Performance of Universal Adhesives: An Updated Systematic Review and Meta-Analysis. J. Adhes. Dent. 2019, 21, 7–26. [Google Scholar] [CrossRef]
- Josic, U.; Mazzitelli, C.; Maravic, T.; Radovic, I.; Jacimovic, J.; Mancuso, E.; Florenzano, F.; Breschi, L.; Mazzoni, A. The influence of selective enamel etch and self-etch mode of universal adhesives’ application on clinical behavior of composite restorations placed on non-carious cervical lesions: A systematic review and meta-analysis. Dent. Mater. 2022, 38, 472–488. [Google Scholar] [CrossRef]
- Josic, U.; Maravic, T.; Mazzitelli, C.; Radovic, I.; Jacimovic, J.; Del Bianco, F.; Florenzano, F.; Breschi, L.; Mazzoni, A. Is clinical behavior of composite restorations placed in non-carious cervical lesions influenced by the application mode of universal adhesives? A systematic review and meta-analysis. Dent. Mater. 2021, 37, e503–e521. [Google Scholar] [CrossRef]
- Breschi, L.; Gobbi, P.; Mazzotti, G.; Falconi, M.; Ellis, T.H.; Stangel, I. High resolution SEM evaluation of dentin etched with maleic and citric acid. Dent. Mater. 2002, 18, 26–35. [Google Scholar] [CrossRef]
- Deyhle, H.; Bunk, O.; Muller, B. Nanostructure of healthy and caries-affected human teeth. Nanomedicine 2011, 7, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, M.; Ohno, H.; Sano, H.; Kaga, M.; Oguchi, H. Degradation patterns of different adhesives and bonding procedures. J. Biomed. Mater. Res. 2003, 66, 324–330. [Google Scholar] [CrossRef]
- Wang, Y.; Spencer, P. Effect of acid etching time and technique on interfacial characteristics of the adhesive-dentin bond using differential staining. Eur. J. Oral Sci. 2004, 112, 293–299. [Google Scholar] [CrossRef]
- Sano, H.; Takatsu, T.; Ciucchi, B.; Horner, J.A.; Matthews, W.G.; Pashley, D.H. Nanoleakage: Leakage within the hybrid layer. Oper Dent 1995, 20, 18–25. [Google Scholar]
- Papadogiannis, D.; Dimitriadi, M.; Zafiropoulou, M.; Gaintantzopoulou, M.D.; Eliades, G. Universal Adhesives: Setting Characteristics and Reactivity with Dentin. Materials 2019, 12, 1720. [Google Scholar] [CrossRef] [Green Version]
- Breschi, L.; Cadenaro, M.; Antoniolli, F.; Sauro, S.; Biasotto, M.; Prati, C.; Tay, F.R.; Di Lenarda, R. Polymerization kinetics of dental adhesives cured with LED: Correlation between extent of conversion and permeability. Dent. Mater. 2007, 23, 1066–1072. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s pioneering acid-etch technique to self-adhering restoratives. A status perspective of rapidly advancing dental adhesive technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar] [CrossRef]
- Yiu, C.K.; Pashley, E.L.; Hiraishi, N.; King, N.M.; Goracci, C.; Ferrari, M.; Carvalho, R.M.; Pashley, D.H.; Tay, F.R. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials 2005, 26, 6863–6872. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, R.M.; Mendonca, J.S.; Santiago, S.L.; Silveira, R.R.; Garcia, F.C.; Tay, F.R.; Pashley, D.H. Effects of HEMA/solvent combinations on bond strength to dentin. J. Dent. Res. 2003, 82, 597–601. [Google Scholar] [CrossRef]
- Cadenaro, M.; Antoniolli, F.; Sauro, S.; Tay, F.R.; Di Lenarda, R.; Prati, C.; Biasotto, M.; Contardo, L.; Breschi, L. Degree of conversion and permeability of dental adhesives. Eur. J. Oral Sci. 2005, 113, 525–530. [Google Scholar] [CrossRef]
- Nunes, T.G.; Garcia, F.C.; Osorio, R.; Carvalho, R.; Toledano, M. Polymerization efficacy of simplified adhesive systems studied by NMR and MRI techniques. Dent. Mater. 2006, 22, 963–972. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H.; Garcia-Godoy, F.; Yiu, C.K. Single-step, self-etch adhesives behave as permeable membranes after polymerization. Part II. Silver tracer penetration evidence. Am. J. Dent. 2004, 17, 315–322. [Google Scholar]
- Tay, F.R.; Pashley, D.H.; Suh, B.; Carvalho, R.; Miller, M. Single-step, self-etch adhesives behave as permeable membranes after polymerization. Part I. Bond strength and morphologic evidence. Am. J. Dent. 2004, 17, 271–278. [Google Scholar]
- Carrilho, M.R.; Tay, F.R.; Donnelly, A.M.; Agee, K.A.; Carvalho, R.M.; Hosaka, K.; Reis, A.; Loguercio, A.D.; Pashley, D.H. Membrane permeability properties of dental adhesive films. J. Biomed. Mater. Res. 2009, 88, 312–320. [Google Scholar] [CrossRef]
- Cruz, J.; Silva, A.; Eira, R.; Sousa, B.; Lopes, M.; Cavalheiro, A. Dentin Permeability and Nanoleakage of Universal Adhesives in Etch-and-rinse vs Self-etch Modes. Oper. Dent. 2021, 46, 293–305. [Google Scholar] [CrossRef]
- Chersoni, S.; Suppa, P.; Grandini, S.; Goracci, C.; Monticelli, F.; Yiu, C.; Huang, C.; Prati, C.; Breschi, L.; Ferrari, M.; et al. In vivo and in vitro permeability of one-step self-etch adhesives. J. Dent. Res. 2004, 83, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Tay, F.R.; Lai, C.N.; Chersoni, S.; Pashley, D.H.; Mak, Y.F.; Suppa, P.; Prati, C.; King, N.M. Osmotic blistering in enamel bonded with one-step self-etch adhesives. J. Dent. Res. 2004, 83, 290–295. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; De Munck, J.; Snauwaert, J.; Coutinho, E.; Poitevin, A.; Yoshida, Y.; Inoue, S.; Peumans, M.; Suzuki, K.; Lambrechts, P.; et al. Monomer-solvent phase separation in one-step self-etch adhesives. J. Dent. Res. 2005, 84, 183–188. [Google Scholar] [CrossRef]
- Burgess, J.O. Materials you connot work without: Refining your tools for treatment. J. Cosmet. Dent. 2013, 28, 94–106. [Google Scholar]
- Spencer, P.; Wang, Y.; Walker, M.P.; Wieliczka, D.M.; Swafford, J.R. Interfacial chemistry of the dentin/adhesive bond. J. Dent. Res. 2000, 79, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Sezinando, A.; Serrano, M.L.; Perez, V.M.; Munoz, R.A.; Ceballos, L.; Perdigao, J. Chemical adhesion of polyalkenoate-based adhesives to hydroxyapatite. J. Adhes. Dent. 2016, 18, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Sezinando, A.; Perdigao, J.; Ceballos, L. Long-term In Vitro Adhesion of Polyalkenoate-based Adhesives to Dentin. J. Adhes. Dent. 2017, 19, 305–316. [Google Scholar] [CrossRef]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Therapeutic effects of novel resin bonding systems containing bioactive glasses on mineral-depleted areas within the bonded-dentine interface. J. Mater. Sci. Mater. Med. 2012, 23, 1521–1532. [Google Scholar] [CrossRef]
- Carvalho, E.M.; Ferreira, P.V.C.; Gutierrez, M.F.; Sampaio, R.F.; Carvalho, C.N.; Menezes, A.S.; Loguercio, A.D.; Bauer, J. Development and characterization of self-etching adhesives doped with 45S5 and niobophosphate bioactive glasses: Physicochemical, mechanical, bioactivity and interface properties. Dent. Mater. 2021, 37, 1030–1045. [Google Scholar] [CrossRef]
- Tatullo, M.; Genovese, F.; Aiello, E.; Amantea, M.; Makeeva, I.; Zavan, B.; Rengo, S.; Fortunato, L. Phosphorene Is the New Graphene in Biomedical Applications. Materials 2019, 12, 2301. [Google Scholar] [CrossRef] [Green Version]
- Tatullo, M.; Zavan, B.; Genovese, F.; Codispoti, B.; Makeeva, I.; Rengo, S.; Fortunato, L.; Spagnuolo, G. Borophene Is a Promising 2D Allotropic Material for Biomedical Devices. Appl. Sci. 2019, 9, 3446. [Google Scholar] [CrossRef] [Green Version]
- Nagarkar, S.; Theis-Mahon, N.; Perdigao, J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J. Biomed. Mater. Res. 2019, 107, 2121–2131. [Google Scholar] [CrossRef]


| Material | Batch Number | Composition |
|---|---|---|
| Clearfil SE Bond Kuraray Noritake (Extra HL) | 5U0640 | Only Bond bottle: 10-MDP, Bis-GMA, hydrophobic dimethacrylate, HEMA, CQ, N,N-diethanol p-toluidine, colloidal silica |
| Prime & Bond Active Dentsply Sirona (PBA) | 2009000399 | Bisacrylamide 1 (25–50%), 10-MDP (10–25%), bisacrylamide 2 (2.5–10%), 4-(dimethylamino) benzonitrile (0.1–1%), PENTA, propan-2-ol (10–25%), water (20%). |
| Scotchbond Universal 3M Oral Care (SBU) | 2019100137 | 10- MDP, dimethacrylate resins, Bis-GMA, HEMA, methacrylatemodified polyalkenoic acid copolymer, CQ, filler, ethanol, water, initiators, silane. |
| Adhesive System | Experimental Groups | Application Mode * |
|---|---|---|
| Prime & Bond Active | Control |
|
| Extra HL |
| |
| Scotchbond Universal | Control |
|
| Extra HL |
|
| Experimental Groups | Immediate (24 h) | 2 Years | |||
|---|---|---|---|---|---|
| Control | Extra HL | Control | Extra HL | ||
| PBA | Sound | 42.9 (4.5) A,B | 48.2 (4.2) A | 19.5 (3.9) D,E | 39.1 (4.1) B |
| Eroded | 32.1 (4.2) C | 38.0 (4.8) B | 15.2 (3.0) E | 35.1 (3.9) B,C | |
| SBU | Sound | 46.5 (4.1) a,b | 51.2 (3.9) a | 21.9 (2.3) e | 45.8 (4.0) a,b |
| Eroded | 28.1 (3.9) c,d | 39.7 (3.5) b,c | 15.6 (3.3) e | 33.2 (3.9) c | |
| Experimental Groups | Immediate (24 h) | 2 Years | |||
|---|---|---|---|---|---|
| Control | Extra HL | Control | Extra HL | ||
| PBA | Sound | 8.7 (1.5) A,B | 6.4 (1.8) A | 16.9 (2.0) B,C | 9.7 (1.5) B |
| Eroded | 19.8 (2.5) C | 14.0 (2.4) B | 26.3 (3.0) D | 18.1 (2.3) C | |
| SBU | Sound | 6.6 (1.7) a | 7.8 (1.5) a | 14.5 (1.4) b | 8.4 (1.7) a |
| Eroded | 19.2 (1.6) c | 13.6 (1.7) b | 30.0 (1.4) d | 16.4 (2.7) b,c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Brito, G.M.A.P.; Silva, D.O.; Macedo, R.F.C.; Ferreira, M.W.C.; Bauer, J.; Pedroso, F.d.B.; Reis, A.; Siqueira, F.S.F.; Loguercio, A.D.; Cardenas, A.F.M. Does the Application of Additional Hydrophobic Resin to Universal Adhesives Increase Bonding Longevity of Eroded Dentin? Polymers 2022, 14, 2701. https://doi.org/10.3390/polym14132701
de Brito GMAP, Silva DO, Macedo RFC, Ferreira MWC, Bauer J, Pedroso FdB, Reis A, Siqueira FSF, Loguercio AD, Cardenas AFM. Does the Application of Additional Hydrophobic Resin to Universal Adhesives Increase Bonding Longevity of Eroded Dentin? Polymers. 2022; 14(13):2701. https://doi.org/10.3390/polym14132701
Chicago/Turabian Stylede Brito, Graça Maria Abreu Pereira, Daniella Oliveira Silva, Rayssa Ferreira Cavaleiro Macedo, Michel Wendlinger Cantanhede Ferreira, Jose Bauer, Flavia de Brito Pedroso, Alessandra Reis, Fabiana Suelen Figuerêdo Siqueira, Alessandro Dourado Loguercio, and Andres Felipe Millan Cardenas. 2022. "Does the Application of Additional Hydrophobic Resin to Universal Adhesives Increase Bonding Longevity of Eroded Dentin?" Polymers 14, no. 13: 2701. https://doi.org/10.3390/polym14132701