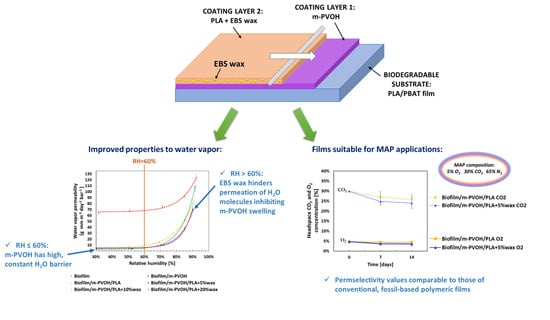
High-Barrier, Biodegradable Films with Polyvinyl Alcohol/Polylactic Acid + Wax Double Coatings: Influence of Relative Humidity on Transport Properties and Suitability for Modified Atmosphere Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of the Double-Coated Films
2.3. Water Vapor Permeability and Modeling
2.4. Gas Permeability Measurements and Permselectivity
2.5. Analysis of Packaging Headspace Gas Composition after MAP
2.6. UV-Vis Spectroscopy
3. Results
3.1. Evaluation and Modeling of the Effect of RH on the Water Vapor Barrier Properties of Coated Films
3.2. Evaluation of the Effect of RH on the Oxygen Barrier Properties of Coated Films
3.3. Carbon Dioxide Barrier Properties, Permselectivity, and Evaluation of Suitability for MAP Application
3.4. Optical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Plastic Pollution is Growing Relentlessly as Waste Management and Recycling Fall Short, Says OECD. Available online: https://www.oecd.org/newsroom/plastic-pollution-is-growing-relentlessly-as-waste-management-and-recycling-fall-short.htm (accessed on 11 September 2023).
- Carullo, D.; Casson, A.; Rovera, C.; Ghaani, M.; Bellesia, T.; Guidetti, R.; Farris, S. Testing a Coated PE-Based Mono-Material for Food Packaging Applications: An in-Depth Performance Comparison with Conventional Multi-Layer Configurations. Food Packag. Shelf Life 2023, 39, 101143. [Google Scholar] [CrossRef]
- Aliotta, L.; Canesi, I.; Lazzeri, A. Study on the Preferential Distribution of Acetyl Tributyl Citrate in Poly(Lactic) Acid-Poly(Butylene Adipate-Co-Terephthalate) Blends. Polym. Test. 2021, 98, 107163. [Google Scholar] [CrossRef]
- Amann, M.; Minge, O. Biodegradability of Poly(vinyl acetate) and Related Polymers. Adv. Polym. Sci. 2012, 245, 137–172. [Google Scholar] [CrossRef]
- Julinová, M.; Vaňharová, L.; Jurča, M. Water-soluble polymeric xenobiotics—Polyvinyl alcohol and polyvinylpyrrolidon—And potential solutions to environmental issues: A brief review. J. Environ. Manag. 2018, 228, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Kawai, F.; Hu, X. Biochemistry of microbial polyvinyl alcohol degradation. Appl. Microbiol. Biotechnol. 2009, 84, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Apicella, A.; Barbato, A.; Garofalo, E.; Incarnato, L.; Scarfato, P. Effect of PVOH/PLA + Wax Coatings on Physical and Functional Properties of Biodegradable Food Packaging Films. Polymers 2022, 14, 935. [Google Scholar] [CrossRef] [PubMed]
- Chuaponpat, N.; Ueda, T.; Ishigami, A.; Kurose, T.; Ito, H. Morphology, Thermal and Mechanical Properties of Co-Continuous Porous Structure of PLA/PVA Blends by Phase Separation. Polymers 2020, 12, 1083. [Google Scholar] [CrossRef]
- Shi, B.; Wideman, G.; Wang, J.H. Improving the Processability of Water-Soluble Films Based on Filled Thermoplastic Polyvinyl Alcohol. Int. Polym. Process. 2012, 27, 231–236. [Google Scholar] [CrossRef]
- Suganthi, S.; Vignesh, S.; Kalyana Sundar, J.; Raj, V. Fabrication of PVA Polymer Films with Improved Antibacterial Activity by Fine-Tuning via Organic Acids for Food Packaging Applications. Appl. Water. Sci. 2020, 10, 100. [Google Scholar] [CrossRef]
- Leneveu-Jenvrin, C.; Apicella, A.; Bradley, K.; Meile, J.; Chillet, M.; Scarfato, P.; Incarnato, L.; Remize, F. Effects of maturity level, steam treatment, or active packaging to maintain the quality of minimally processed mango (Mangifera indica cv. José). J. Food Process. Preserv. 2021, 45, e15600. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.K. Modified Atmosphere Packaging of Fruits and Vegetables for Extending Shelf-Life: A Review. J. Food Process. Preserv. 2009, 3, 1–31. [Google Scholar]
- Apicella, A.; Scarfato, P.; Di Maio, L.; Incarnato, L. Oxygen Absorption Data of Multilayer Oxygen Scavenger-Polyester Films with Different Layouts. Data Brief 2018, 19, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Florit, F.; Fiorati, A.; Ghisoni, F.; Pozzoli, G.; Rota, R.; De Nardo, L. Development of a Generalised Equilibrium Modified Atmosphere Model and Its Application to the Taleggio Cheese. J. Food Eng. 2022, 315, 110765. [Google Scholar] [CrossRef]
- Apicella, A.; Scarfato, P.; Di Maio, L.; Garofalo, E.; Incarnato, L. Evaluation of Performance of PET Packaging Films Based on Different Copolyester O2-Scavengers. In Proceedings of the 9th International Conference on “Times of Polymers and Composites”: From Aerospace to Nanotechnology, Ischia, Italy, 17–21 June 2018; p. 020130. [Google Scholar]
- Shen, Z.; Kwon, S.; Oh, K.; Abhari, A.R.; Lee, H.L. Facile Fabrication of Hydrophobic Cellulosic Paper with Good Barrier Properties via PVA/AKD Dispersion Coating. Nord. Pulp Pap. Res. J. 2019, 34, 516–524. [Google Scholar] [CrossRef]
- Yue, S.; Wang, S.; Hana, D.; Huang, S.; Sun, L.; Xiao, M.; Meng, Y. Polyvinyl Alcohol/Montmorillonite Nanocomposite Coated Biodegradable Films with Outstanding Barrier Properties. ES Mater. Manuf. 2023, 20, 834. [Google Scholar] [CrossRef]
- Andrade, J.; González-Martínez, C.; Chiralt, A. Antimicrobial PLA-PVA Multilayer Films Containing Phenolic Compounds. Food Chem. 2022, 375, 131861. [Google Scholar] [CrossRef] [PubMed]
- Anukiruthika, T.; Sethupathy, P.; Wilson, A.; Kashampur, K.; Moses, J.A.; Anandharamakrishnan, C. Multilayer Packaging: Advances in Preparation Techniques and Emerging Food Applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1156–1186. [Google Scholar] [CrossRef]
- Ge, L.; Zhao, Y.; Mo, T.; Li, J.; Li, P. Immobilization of Glucose Oxidase in Electrospun Nanofibrous Membranes for Food Preservation. Food Control 2012, 26, 188–193. [Google Scholar] [CrossRef]
- Ullah, S.; Hashmi, M.; Shi, J.; Kim, I.S. Fabrication of Electrospun PVA/Zein/Gelatin Based Active Packaging for Quality Maintenance of Different Food Items. Polymers 2023, 15, 2538. [Google Scholar] [CrossRef]
- Kim, H.; Panda, P.K.; Sadeghi, K.; Seo, J. Poly (Vinyl Alcohol)/Hydrothermally Treated Tannic Acid Composite Films as Sustainable Antioxidant and Barrier Packaging Materials. Prog. Org. Coat. 2023, 174, 107305. [Google Scholar] [CrossRef]
- Nguyen, S.V.; Lee, B.-K. Multifunctional Nanocomposite Based on Polyvinyl Alcohol, Cellulose Nanocrystals, Titanium Dioxide, and Apple Peel Extract for Food Packaging. Int. J. Biol. Macromol. 2023, 227, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zhou, C.; Yang, Z.; Wang, C.; He, Z.; Liu, Y.; Song, S.; Chen, Y.; Xie, M.; Li, P. Preparation and Characterization of Functionalized Chitosan/Polyvinyl Alcohol Composite Films Incorporated with Cinnamon Essential Oil as an Active Packaging Material. Int. J. Biol. Macromol. 2023, 235, 123914. [Google Scholar] [CrossRef] [PubMed]
- Suhag, A.; Biswas, K.; Singh, S.; Kulshreshtha, A. Crosslinking Effect on Polyvinyl Alcohol Resin for Barrier Properties of Barrier Biaxial Orientation Films. Prog. Org. Coat. 2022, 163, 106662. [Google Scholar] [CrossRef]
- Gigante, V.; Panariello, L.; Coltelli, M.-B.; Danti, S.; Obisesan, K.A.; Hadrich, A.; Staebler, A.; Chierici, S.; Canesi, I.; Lazzeri, A.; et al. Liquid and Solid Functional Bio-Based Coatings. Polymers 2021, 13, 3640. [Google Scholar] [CrossRef] [PubMed]
- Apicella, A.; Scarfato, P.; Di Maio, L.; Incarnato, L. Sustainable Active PET Films by Functionalization with Antimicrobial Bio-Coatings. Front. Mater. 2019, 6, 243. [Google Scholar] [CrossRef]
- Mesgari, M.; Aalami, A.H.; Sathyapalan, T.; Sahebkar, A. A Comprehensive Review of the Development of Carbohydrate Macromolecules and Copper Oxide Nanocomposite Films in Food Nanopackaging. Bioinorg. Chem. Appl. 2022, 2022, 7557825. [Google Scholar] [CrossRef] [PubMed]
- Janjarasskul, T.; Tananuwong, K.; Phupoksakul, T.; Thaiphanit, S. Fast Dissolving, Hermetically Sealable, Edible Whey Protein Isolate-Based Films for Instant Food and/or Dry Ingredient Pouches. LWT 2020, 134, 110102. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Tananuwong, K. Role of Whey Proteins in Food Packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019; p. 97B80081005965224000. ISBN 978-0-08-100596-5. [Google Scholar]
- Buonocore, G.G.; Conte, A.; Nobile, M.A.D. Use of a Mathematical Model to Describe the Barrier Properties of Edible Films. J. Food Sci. 2005, 70, E142–E147. [Google Scholar] [CrossRef]
- Nobile, M.A.; Buonocore, G.G.; Altieri, C.; Battaglia, G.; Nicolais, L. Modeling the Water Barrier Properties of Nylon Film Intended for Food Packaging Applications. J. Food Sci. 2003, 68, 1334–1340. [Google Scholar] [CrossRef]
- Apicella, A.; Scarfato, P.; Incarnato, L. Tailor-Made Coextruded Blown Films Based on Biodegradable Blends for Hot Filling and Frozen Food Packaging. Food Packag. Shelf Life 2023, 3, 101096. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y.; Han, N.; Liu, S. Water and Gas Barrier Properties of Polyvinyl Alcohol (PVA)/Starch (ST)/ Glycerol (GL)/Halloysite Nanotube (HNT) Bionanocomposite Films: Experimental Characterisation and Modelling Approach. Compos. Part B Eng. 2019, 174, 107033. [Google Scholar] [CrossRef]
- Mo, C.; Yuan, W.; Lei, W.; Shijiu, Y. Effects of Temperature and Humidity on the Barrier Properties of Biaxially-Oriented Polypropylene and Polyvinyl Alcohol Films. J. Appl. Packag. Res. 2014, 6, 40–46. [Google Scholar] [CrossRef]
- DEUREX X. 2010 M Technical Information and Material Safety Data Sheet. Available online: https://www.deurex.com/productsearch/DEUREX-X-2010-M/ (accessed on 26 September 2023).
- General Information on N,N′-Ethylenedi(Stearamide), ECHA Database. Available online: https://echa.europa.eu/it/registration-dossier/-/registered-dossier/12372 (accessed on 26 September 2023).
- Robertson, G.L. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439862421. [Google Scholar]
- Wang, L.; Liu, X.; Qi, P.; Sun, J.; Jiang, S.; Li, H.; Gu, X.; Zhang, S. Enhancing the thermostability, UV shielding, and antimicrobial activity of transparent chitosan film by carbon quantum dots containing N/P. Carbohydr. Polym. 2022, 278, 118957. [Google Scholar] [CrossRef] [PubMed]
- Lasoski, S.W.; Cobbs, W.H. Moisture Permeability of Polymers. I. Role of Crystallinity and Orientation. J. Polym. Sci. 1959, 36, 21–33. [Google Scholar] [CrossRef]
- Melnikova, S.D.; Larin, S.V. Influence of Polymer Compatibility and Layer Thickness on the Structural and Thermophysical Properties of Polymer Multilayer Films. J. Polym. Sci. 2023, 61, 671–684. [Google Scholar] [CrossRef]
- Swarbrick, J.; Amann, A.H. Moisture Permeation through Polymer Films. J. Pharm. Pharmacol. 2011, 20, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and Oxygen Barrier Properties of Cellulose Nanomaterial-Based Films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Pietrosanto, A.; Scarfato, P.; Di Maio, L.; Nobile, M.R.; Incarnato, L. Evaluation of the Suitability of Poly(Lactide)/Poly(Butylene-Adipate-Co-Terephthalate) Blown Films for Chilled and Frozen Food Packaging Applications. Polymers 2020, 12, 804. [Google Scholar] [CrossRef]
- Apicella, A.; Adiletta, G.; Di Matteo, M. Incarnato Loredana Valorization of Olive Industry Waste Products for Development of New Eco-Sustainable, Multilayer Antioxidant Packaging for Food Preservation. Chem. Eng. Trans. 2019, 75, 85–90. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Kuraray Europe GmbH. Basic Physical Properties of PVOH Resin. Available online: https://www.kuraray.eu/fileadmin/product_groups/polyvinylalcohol/downloads/kuraray_poval_basic_physical_properties_web.pdf (accessed on 11 September 2023).
- Johansson, C.; Clegg, F. Hydrophobically Modified Poly(Vinyl Alcohol) and Bentonite Nanocomposites Thereof: Barrier, Mechanical, and Aesthetic Properties. J. Appl. Polym. Sci. 2015, 132, 41737. [Google Scholar] [CrossRef]
- Trifol, J.; Plackett, D.; Szabo, P.; Daugaard, A.E.; Giacinti Baschetti, M. Effect of Crystallinity on Water Vapor Sorption, Diffusion, and Permeation of PLA-Based Nanocomposites. ACS Omega 2020, 5, 15362–15369. [Google Scholar] [CrossRef] [PubMed]
- Messin, T.; Marais, S.; Follain, N.; Guinault, A.; Gaucher, V.; Delpouve, N.; Sollogoub, C. Biodegradable PLA/PBS Multinanolayer Membrane with Enhanced Barrier Performances. J. Membr. Sci. 2020, 598, 117777. [Google Scholar] [CrossRef]
- Sonchaeng, U.; Iñiguez-Franco, F.; Auras, R.; Selke, S.; Rubino, M.; Lim, L.-T. Poly(Lactic Acid) Mass Transfer Properties. Prog. Polym. Sci. 2018, 86, 85–121. [Google Scholar] [CrossRef]
- Chow, W.S.; Lim, S.R. Effects of N,N′-Ethylenebis(Stearamide) on the Properties of Poly(Ethylene Terephthalate)/Organo-Montmorillonite Nanocomposite. Polym. Plast. Technol. Eng. 2013, 52, 626–633. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.-W.; Hu, C.-Y.; Wang, L.; Wang, P.-L. Vapor permeation in flexible packaging films. Polym. Mater. Sci. Eng. 2009, 25, 42–45. [Google Scholar]
- The Role of Carbon Dioxide in Modified Atmosphere. Available online: https://blog.masterpackgroup.com/the-role-of-carbon-dioxide-in-modified-atmosphere (accessed on 26 September 2023).
- Air Liquid. Quale Atmosfera Protettiva per i Prodotti Secchi? Available online: https://it.airliquide.com/soluzioni/confezionamento-atmosfera-protettiva-map/quale-atmosfera-protettiva-i-prodotti-secchi (accessed on 11 September 2023).
- Ludwiczak, J.; Frąckowiak, S.; Leluk, K. Study of Thermal, Mechanical and Barrier Properties of Biodegradable PLA/PBAT Films with Highly Oriented MMT. Materials 2021, 14, 7189. [Google Scholar] [CrossRef]
- Nomura, K.; Terwilliger, P. Investigation on Compatibility of PLA/PBAT Blends Modified by Epoxy-Terminated Branched Polymers through Chemical Micro-Crosslinking. Spec. Matrices 2019, 7, 1–19. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Waterhouse, G.I.N.; Gong, R.; Xie, J.; Zhang, K.; Xu, J. Optimizing Interfacial Adhesion in PBAT/PLA Nanocomposite for Biodegradable Packaging Films. Food Chem. 2021, 334, 127487. [Google Scholar] [CrossRef]
- Klepić, M.; Setničková, K.; Lanč, M.; Žák, M.; Izák, P.; Dendisová, M.; Fuoco, A.; Jansen, J.C.; Friess, K. Permeation and Sorption Properties of CO2-Selective Blend Membranes Based on Polyvinyl Alcohol (PVA) and 1-Ethyl-3-Methylimidazolium Dicyanamide ([EMIM][DCA]) Ionic Liquid for Effective CO2/H2 Separation. J. Membr. Sci. 2020, 597, 117623. [Google Scholar] [CrossRef]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the Barrier Properties of PLA: A State-of-the-Art Review for Food Packaging Applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef] [PubMed]
- Faraj, H.; Follain, N.; Sollogoub, C.; Almeida, G.; Chappey, C.; Marais, S.; Tencé-Girault, S.; Gouanvé, F.; Espuche, E.; Domenek, S. Gas Barrier Properties of Polylactide/Cellulose Nanocrystals Nanocomposites. Polym. Test. 2022, 113, 107683. [Google Scholar] [CrossRef]
- Alves, R.M.V.; De Luca Sarantópoulos, C.I.G.; Van Dender, A.G.F.; De Assis Fonseca Faria, J. Stability of Sliced Mozzarella Cheese in Modified-Atmosphere Packaging. J. Food Prot. 1996, 59, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fandos, E.; Sanz, S.; Olarte, C. Microbiological, Physicochemical and Sensory Characteristics of Cameros Cheese Packaged under Modified Atmospheres. Food Microbiol. 2000, 17, 407–414. [Google Scholar] [CrossRef]
- Joles, D.W.; Cameron, A.C.; Shirazi, A.; Petracek, P.D.; Beaudry, R.M. Modified-Atmosphere Packaging of ‘Heritage’ Red Raspberry Fruit: Respiratory Response to Reduced Oxygen, Enhanced Carbon Dioxide, and Temperature. J. Am. Soc. Hortic. Sci. 1994, 119, 540–545. [Google Scholar] [CrossRef]
- Al-Ati, T.; Hotchkiss, J.H. The Role of Packaging Film Permselectivity in Modified Atmosphere Packaging. J. Agric. Food Chem. 2003, 51, 4133–4138. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.V.; Caleb, O.J. Modeling MAP of Fruits and Vegetables. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2017; p. B9780081005965214000. ISBN 978-0-08-100596-5. [Google Scholar]
- Caleb, O.J.; Opara, U.L.; Witthuhn, C.R. Modified Atmosphere Packaging of Pomegranate Fruit and Arils: A Review. Food Bioprocess Technol. 2012, 5, 15–30. [Google Scholar] [CrossRef]
- Gore, A.H.; Prajapat, A.L. Biopolymer Nanocomposites for Sustainable UV Protective Packaging. Front. Mater. 2022, 9, 855727. [Google Scholar] [CrossRef]





| Sample Film | Wax Concentration [% w/wPLA] | Thickness of the m-PVOH Coating [μm] | Thickness of the PLA-Based Coatings [μm] | Total Thickness [μm] |
|---|---|---|---|---|
| Biofilm | 0 | - | - | 22 ± 2 |
| Biofilm/m-PVOH | 0 | 10 ± 1 | - | 32 ± 2 |
| Biofilm/m-PVOH/PLA | 0 | 10 ± 1 | 6 ± 2 | 38 ± 4 |
| Biofilm/m-PVOH/PLA + 5%wax | 5 | 10 ± 1 | 6 ± 1 | 38 ± 5 |
| Biofilm/m-PVOH/PLA + 10%wax | 10 | 10 ± 1 | 7 ± 1 | 39 ± 4 |
| Biofilm/m-PVOH/PLA + 20%wax | 20 | 10 ± 1 | 8 ± 1 | 40 ± 3 |
| Sample Film | PWV [g mm/ m2 d bar] | ΔPWV% | ||||||
|---|---|---|---|---|---|---|---|---|
| 30% RH | 50% RH | 70% RH | 85% RH | 90% RH | 70% RH | 85% RH | 90% RH | |
| Biofilm | 64.0 ± 2.1 | 67.9 ± 2.9 | 75.0 ± 3.0 | 92.0 ± 1.8 | 124.0 ± 6.0 | - | - | - |
| Biofilm/m-PVOH | 3.4 ± 0.2 | 3.5 ± 0.5 | 10.3 ± 1.8 | 52.8 ± 0.7 | 107.3 ± 7.8 | - | - | - |
| Biofilm/m-PVOH/PLA | 6.0 ± 0.2 | 5.7 ± 0.4 | 15.1 ± 1.1 | 54.0 ± 1.9 | 82.2 ± 4.2 | - | - | - |
| Biofilm/m-PVOH/PLA + 5%wax | 4.5 ± 0.8 | 4.3 ± 0.1 | 9.5 ± 0.6 | 37.0 ± 2.1 | 69.6 ± 0.7 | 37.1 | 31.5 | 15.3 |
| Biofilm/m-PVOH/PLA + 10%wax | 4.7 ± 0.7 | 4.9 ± 0.5 | 9.9 ± 0.6 | 37.1 ± 0.9 | 71.8 ± 0.9 | 34.4 | 31.3 | 12.7 |
| Biofilm/m-PVOH/PLA + 20%wax | 4.5 ± 0.6 | 4.6± 0.3 | 10.9 ± 0.7 | 35.8 ± 2.9 | 70.3 ± 4.5 | 27.8 | 33.7 | 14.5 |
| Sample Film | a × 10−3 | b | c |
|---|---|---|---|
| Biofilm | 5.035 | 10.133 | 65.948 |
| Biofilm/m-PVOH | 2.134 | 11.813 | 2.187 |
| Biofilm/m-PVOH/PLA | 15.727 | 9.409 | 4.750 |
| Biofilm/m-PVOH/PLA + 5%wax | 1.108 | 12.153 | 4.036 |
| Biofilm/m-PVOH/PLA + 10%wax | 20.715 | 14.075 | 4.978 |
| Biofilm/m-PVOH/PLA + 20%wax | 896.146 | 15.006 | 5.190 |
| Sample Film | [cm3 mm/m2 day bar] | Permselectivity PCO2/PO2 |
|---|---|---|
| Biofilm | 305 ± 79 | 8.0 |
| Biofilm/m-PVOH | 0.37 ± 0.11 | 3.8 |
| Biofilm/m-PVOH/PLA | 0.46 ± 0.14 | 2.1 |
| Biofilm/m-PVOH/PLA + 5%wax | 0.44 ± 0.30 | 3.7 |
| Biofilm/m-PVOH/PLA + 10%wax | 0.45 ± 0.18 | 4.9 |
| Biofilm/m-PVOH/PLA + 20%wax | 0.46 ± 0.10 | 4.3 |
| Sample Film | TR [%] | UVA-Blocking % | UVB-Blocking % |
|---|---|---|---|
| Biofilm | 9.6 | 96.7 | 99.7 |
| Biofilm/m-PVOH | 17.0 | 92.0 | 99.2 |
| Biofilm/m-PVOH/PLA | 17.1 | 92.0 | 99.2 |
| Biofilm/m-PVOH/PLA + 5%wax | 14.3 | 93.3 | 99.3 |
| Biofilm/m-PVOH/PLA + 10%wax | 12.2 | 94.6 | 99.5 |
| Biofilm/m-PVOH/PLA + 20%wax | 9.1 | 96.6 | 99.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbato, A.; Apicella, A.; Malvano, F.; Scarfato, P.; Incarnato, L. High-Barrier, Biodegradable Films with Polyvinyl Alcohol/Polylactic Acid + Wax Double Coatings: Influence of Relative Humidity on Transport Properties and Suitability for Modified Atmosphere Packaging Applications. Polymers 2023, 15, 4002. https://doi.org/10.3390/polym15194002
Barbato A, Apicella A, Malvano F, Scarfato P, Incarnato L. High-Barrier, Biodegradable Films with Polyvinyl Alcohol/Polylactic Acid + Wax Double Coatings: Influence of Relative Humidity on Transport Properties and Suitability for Modified Atmosphere Packaging Applications. Polymers. 2023; 15(19):4002. https://doi.org/10.3390/polym15194002
Chicago/Turabian StyleBarbato, Antonio, Annalisa Apicella, Francesca Malvano, Paola Scarfato, and Loredana Incarnato. 2023. "High-Barrier, Biodegradable Films with Polyvinyl Alcohol/Polylactic Acid + Wax Double Coatings: Influence of Relative Humidity on Transport Properties and Suitability for Modified Atmosphere Packaging Applications" Polymers 15, no. 19: 4002. https://doi.org/10.3390/polym15194002