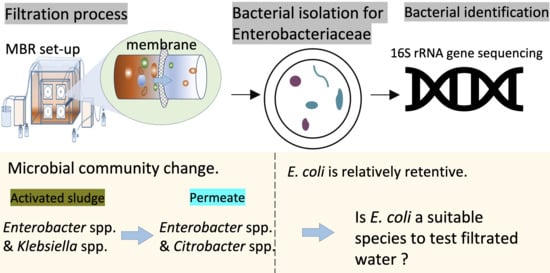
Constituents of Coliform Species Contained in the Permeate of Microfiltration Membranes in Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Filtration of Activated Sludge
2.2. Permeate Collecting and Cultivation of Enterobacteriaceae
2.3. Identification of Isolated Bacteria
2.3.1. DNA Extraction
2.3.2. PCR Amplification, Purification, and Sequencing
2.4. Size Measure and Gram Staining of Target Bacteria
3. Results
3.1. Effect of Pore Size on the Bacterial Presence in the Permeate
3.2. Filtration Volume Flux and Time-Dependent Bacterial Leakage
3.3. Bacterial Community Change of Enterobacteriaceae during MBR Process
3.4. Detected Species in the Permeate of Specific Membrane
3.5. Bacterial Sizes Found in the Permeate
4. Discussion
4.1. Factors Affecting Bacterial Penetration through Microfiltration Membranes
4.2. Bacterial Community Change by Filtration
4.3. Health-Related Risks of Water Treated by Microfiltration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Judd, S.J. The status of industrial and municipal effluent treatment with membrane bioreactor technology. J. Chem. Eng. 2016, 305, 37–45. [Google Scholar] [CrossRef]
- Xiao, K.; Xu, Y.; Liang, S.; Lei, T.; Sun, J.; Wen, X.; Zhang, H.; Chen, C.; Huang, X. Engineering application of membrane bioreactor for wastewater treatment in China: Current state and future prospect. Front. Environ. Sci. Eng. 2014, 8, 805–819. [Google Scholar] [CrossRef]
- Ren, Y.; Ngo, H.H.; Guo, W.; Wang, D.; Peng, L.; Ni, B.J.; Wei, W.; Liu, Y. New perspectives on microbial communities and biological nitrogen removal processes in wastewater treatment systems. Bioresour. Technol. 2020, 297, 122491. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Rajta, A.; Setial, H.; Bhatia, R. Simultaneous nitrification–denitrification by phosphate accumulating microorganisms. World J. Microbiol. Biotechnol. 2020, 36, 151. [Google Scholar] [CrossRef]
- Dorofeev, A.G.; Nikolaev, Y.A.; Mardanov, A.V.; Pimenov, N.V. Role of phosphate-accumulating bacteria in biological phosphorus removal from wastewater. Appl. Biochem. Microbiol. 2020, 56, 1–14. [Google Scholar] [CrossRef]
- Qiu, G.; Zuniga-Montanez, R.; Law, Y.; Thi, S.S.; Nguyen, T.Q.N.; Eganathan, K.; Liu, X.; Nielsen, P.H.; Williams, R.B.H.; Wuertz, S. Polyphosphate-accumulating organisms in full-scale tropical wastewater treatment plants use diverse carbon sources. Water Res. 2019, 149, 496–510. [Google Scholar] [CrossRef]
- Diep, P.; Mahadevan, R.; Yakunin, A.F. Heavy metal removal by bioaccumulation using genetically engineered microorganisms. Front. Bioeng. Biotechnol. 2018, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Sipma, J.; Osuna, B.; Collado, N.; Monclús, H.; Ferrero, G.; Comas, J.; Rodriguez-Roda, I. Comparison of removal of pharmaceuticals in MBR and activated sludge systems. Desalination 2010, 250, 653–659. [Google Scholar] [CrossRef]
- Anand, U.; Reddy, B.; Singh, V.K.; Singh, A.K.; Kesari, K.K.; Tripathi, P.; Kumar, P.; Tripathi, V.; Simal-Gandara, J. Potential environmental and human health risks caused by antibiotic-resistant bacteria (ARB), antibiotic resistance genes (ARGs) and emerging contaminants (ECs) from municipal solid waste (MSW) landfill. Antibiotics 2021, 10, 374. [Google Scholar] [CrossRef]
- Dolar, D.; Gros, M.; Rodriguez-Mozaz, S.; Moreno, J.; Comas, J.; Rodriguez-Roda, I.; Barceló, D. Removal of emerging contaminants from municipal wastewater with an integrated membrane system, MBR–RO. J. Hazard. Mater. 2012, 239, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Yamamoto, K. Membrane biological reactors. In Treatise on Water Science; Wilderer, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 571–613. [Google Scholar]
- Harb, M.; Hong, P.Y. Molecular-based detection of potentially pathogenic bacteria in membrane bioreactor (MBR) systems treating municipal wastewater: A case study. Environ. Sci. Pollut. Res. Int. 2017, 24, 5370–5380. [Google Scholar] [CrossRef] [PubMed]
- Helling, A.; Kubicka, A.; Schaap, I.A.T.; Polakovic, M.; Hansmann, B.; Thiess, H.; Strube, J.; Thom, V. Passage of soft pathogens through microfiltration membranes scales with transmembrane pressure. J. Membr. Sci. 2017, 522, 292–302. [Google Scholar] [CrossRef]
- Hirani, Z.M.; Bukhari, Z.; Oppenheimer, J.; Jjemba, P.; LeChevallier, M.W.; Jacangelo, J.G. Characterization of effluent water qualities from satellite membrane bioreactor facilities. Water Res. 2013, 47, 5065–5075. [Google Scholar] [CrossRef] [PubMed]
- Stockner, J.G.; Klut, M.E.; Cochlan, W.P. Leaky filters: A warning to aquatic ecologists. Can. J. Fish. Aquat. Sci. 1990, 47, 16–23. [Google Scholar] [CrossRef]
- Arthur, G.; Clémence, C.; Christine, R.; Patrice, B.; Etienne, D.; Christel, C. Bacteria transfer by deformation through microfiltration membrane. J. Membr. Sci. 2017, 523, 446–455. [Google Scholar]
- Velimirov, B. Nanobacteria, ultramicrobacteria and starvation forms: A search for the smallest metabolizing bacterium. Microbes Environ. 2001, 16, 67–77. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hammes, F.; Düggelin, M.; Egli, T. Influence of size, shape, and flexibility on bacterial passage through micropore membrane filters. Environ. Sci. Technol. 2008, 42, 6749–6754. [Google Scholar] [CrossRef] [PubMed]
- Lebleu, N.; Roques, C.; Aimar, P.; Causserand, C. Role of the cell-wall structure in the retention of bacteria by microfiltration membranes. J. Membr. Sci. 2009, 326, 178–185. [Google Scholar] [CrossRef]
- Hood, M.A.; MacDonell, M.T. Distribution of ultramicrobacteria in a gulf coast estuary and induction of ultramicrobacteria. Microb. Ecol. 1987, 14, 113–127. [Google Scholar] [CrossRef]
- Ghuneim, L.A.J.; Jones, D.L.; Golyshin, P.N.; Golyshina, O.V. Nano-sized and filterable bacteria and archaea: Biodiversity and function. Front. Microbiol. 2018, 9, 1971. [Google Scholar] [CrossRef]
- Suzina, N.E.; Duda, V.I.; Esikova, T.Z.; Shorokhova, A.P.; Gafarov, A.B.; Oleinikov, R.R.; Akimov, V.N.; Abashina, T.N.; Polivtseva, V.N.; Boronin, A.M. Novel ultramicrobacteria, strains NF4 and NF5, of the genus Chryseobacterium: Facultative epibionts of Bacillus subtilis. Microbiology 2011, 80, 535–548. [Google Scholar] [CrossRef]
- Luef, B.; Frischkorn, K.R.; Wrighton, K.C.; Holman, H.Y.N.; Birarda, G.; Thomas, B.C.; Singh, A.; Williams, K.H.; Siegerist, C.E.; Tringe, S.G.; et al. Diverse uncultivated ultra-small bacterial cells in groundwater. Nat. Commun. 2015, 6, 6372. [Google Scholar] [CrossRef]
- Maejima, Y.; Kushimoto, K.; Muraguchi, Y.; Fukuda, K.; Miura, T.; Yamazoe, A.; Kimbara, K.; Shintani, M. Proteobacteria and Bacteroidetes are major phyla of filterable bacteria passing through 0.22 μm pore size membrane filter, in Lake Sanaru, Hamamatsu, Japan. Biosci. Biotechnol. Biochem. 2018, 82–87, 1260–1263. [Google Scholar] [CrossRef]
- Helling, A.; Grote, C.; Büning, D. Ulbricht Mathias, Wessling Matthias, Polakovic Milan, Thom Volkmar. Influence of flow alterations on bacteria retention during microfiltration. J. Membr. Sci. 2019, 575, 147–159. [Google Scholar] [CrossRef]
- Cronholm, K.; Bates, S.; Nguyen, N.; Leahy, A.; Blanchard, M.; Lentine, K.R. Validation of a microbiological method using Acholeplasma laidlawii for assessing performance of microporous membranes for mycoplasma clearance. PDA J. Pharm. Sci. Technol. 2009, 63, 438–461. [Google Scholar]
- Folmsbee, M.; Howard, G.; McAlister, M. Nutritional effects of culture media on mycoplasma cell size and removal by filtration. Biologicals 2010, 38, 214–217. [Google Scholar] [CrossRef]
- Zhou, S.; Ninoseki, M.; Kusaba, A.; Nakagawa, K.; Urase, T. Bacterial species identified in the filtrate of microfiltration membranes in the separation of activated sludge. JWET 2021, 19, 294–301. [Google Scholar] [CrossRef]
- Li, D.L.; Liu, S.Y. Water Quality Monitoring in Aquaculture. In Water Quality Monitoring and Management; Academic Press: Cambridge, MA, USA, 2019; pp. 303–328. [Google Scholar]
- Patel, A.K.; Singhania, R.R.; Pandey, A.; Joshi, V.K.; Nigam, P.S.; Soccol, C.R. Introduction Enterobacteriaceae, Coliforms and E. coli. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Elsevier Inc.: Exeter Devon, UK, 2014; Volume 1, pp. 659–666. [Google Scholar]
- Mermin, J.H.; Villar, R.; Carpenter, J.; Roberts, L.; Samaridden, A.; Gasanova, L.; Lomakina, S.; Bopp, C.; Hutwagner, L.; Mead, P.; et al. A massive epidemic of multidrug-resistant typhoid fever in tajikistan associated with consumption of municipal water. J. Infect. Dis. 1999, 179, 1416–1422. [Google Scholar] [CrossRef]
- Weissman, J.B.; Craun, G.F.; Lawrence, D.N.; Pollard, R.A.; Saslavv, M.S.; Gangarosa, E.J. An epidemic of gastroenteritis traced to a contaminated public water supply. Am. J. Epidemiol. 1976, 103, 391–398. [Google Scholar] [CrossRef]
- Laborde, D.J.; Weigle, K.A.; Weber, D.J.; Kotch, J.B. Effect of fecal contamination on diarrheal illness rates in day-care centers. Am. J. Epidemiol. 1993, 138, 243–255. [Google Scholar] [CrossRef]
- Greig, J.D.; Todd, E.C.D.; Bartleson, C.; Michaels, B. Infective Doses and Pathogen Carriage. In Proceedings of the USDA 2010 Food Safety Education Conference, Atlanta, GA, USA, 25 March 2010. [Google Scholar]
- Khatoon, A.; Pirzada, Z.A. Bacteriological quality of bottled water brands in Karachi. Pakistan. Biologia (Pakistan) 2010, 56, 137–143. [Google Scholar]
- Apel, P.Y.; Blonskaya, I.V.; Dmitriev, S.N.; Orelovitch, O.L.; Sartowska, B. Structure of polycarbonate track-etch membranes: Origin of the “paradoxical” pore shape. J. Membr. Sci. 2006, 282, 393–400. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA Sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackebrandt., E., Goodfellow, M., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 1991; pp. 115–175. [Google Scholar]
- Gram Staining. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562156/ (accessed on 14 August 2023).
- Lee, A.; McVey, J.; Faustino, P.; Lute, S.; Sweeney, N.; Pawar, V.; Khan, M.; Brorson, K.; Hussong, D. Use of Hydrogenophaga pseudoflava penetration to quantitatively assess the impact of filtration parameters for 0.2-micrometer-pore-size filters. Appl. Environ. Microbiol. 2010, 76, 695–700. [Google Scholar] [CrossRef]
- Percival, S.L.; Williams, D.W. Escherichia coli. In Microbiology of Waterborne Diseases, 2nd ed.; Percival, S.L., Yates, M.V., Williams, D.W., Chalmers, R.M., Gray, N.F., Eds.; Elsevier Ltd.: Amsterdam, The Netherland; Academic Press: Cambridge, MA, USA, 2014; pp. 89–117. [Google Scholar]
- Kim, K.; Baltus, R.E.; Chellam, S. Rejection and fouling of track-etched microfiltration membranes by Acholeplasma laidlawii: Clues to mycoplasma behavior during “sterile” dead-end filtration. J. Membr. Sci. 2023, 685, 121925. [Google Scholar] [CrossRef]
- Flemming, H.C.; Percival, S.L.; Walker, J.T. Contamination potential of biofilms in water distribution systems. Water Supply 2002, 2, 271–280. [Google Scholar] [CrossRef]
- Abebe, G.M. The role of bacterial biofilm in antibiotic resistance and food contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef]
- Liu, J.; Li, B.; Wang, Y.Y.; Zhang, G.J.; Jiang, X.T.; Li, X.Y. Passage and community changes of filterable bacteria during microfiltration of a surface water supply. Environ. Int. 2019, 131, 104998. [Google Scholar] [CrossRef]
- Bereschenko, L.A.; Stams, A.J.M.; Heilig, G.H.J.; Euverink, G.J.W.; Nederlof, M.M.; Van Loosdrecht, M.C.M. Investigation of microbial communities on reverse osmosis membranes used for process water production. Water Sci Technol. 2007, 55, 181–190. [Google Scholar] [CrossRef]
- Guo, M.; Huang, J.; Hu, H.; Liu, W. Growth and repair potential of three species of bacteria in reclaimed wastewater after UV disinfection. Biomed. Environ. Sci. 2011, 24, 400–407. [Google Scholar]
- Silveira, A.B.D.; Bechtlufft, M.D.P.; Van Der Sand, S.T.; Corçã, G. Evaluation of the activity of disinfectants against coliform bacteria group strains isolated from a sewage treatment plant (ETE-Ipanema). Acta Sci. Vet. 2006, 34, 71–76. [Google Scholar] [CrossRef]
- Cho, M.; Kim, J.; Kim, J.Y.; Yoon, J.; Kim, J.H. Mechanisms of Escherichia coli inactivation by several disinfectants. Water Res. 2010, 44, 3410–3418. [Google Scholar] [CrossRef] [PubMed]





| Run# | Number of Pieces of Membranes Examined | Coliform Numbers in the Feed-Activated Sludge | Filtration Process Duration | ||||
|---|---|---|---|---|---|---|---|
| 0.2 µm | 0.4 µm | 0.8 µm | E. coli (CFU/mL) | Other Enterobacteriaceae (CFU/mL) | Proportion of E. coli | ||
| R1 | 1 | 1 | 1 | 7.0 × 101 | 1.0 × 103 | 6.5% | 24 h |
| R2 | 1 | 1 | 1 | 2.6 × 103 | 1.1 × 104 | 19.1% | 24 h |
| R3 | 1 | 2 | 1 | 9.5 × 103 | 1.5 × 104 | 38.8% | 168 h |
| R4 | 1 | 2 | 1 | 1.3 × 104 | 4.3 × 104 | 23.2% | 120 h |
| R5 | 1 | 2 | 1 | 1.9 × 104 | 2.3 × 104 | 45.2% | 120 h |
| Run# | Numbers of Mauve-Colored (Blue-Colored) Colonies, CFU/mL | |||
|---|---|---|---|---|
| 0.2 µm | 0.4 µm (1) | 0.4 µm (2) | 0.8 µm | |
| R1 | <2.5 (<2.5) | <2.5 (<2.5) | Not examined | 5 (<2.5) |
| R2 | <2.5 (<2.5) | <2.5 (<2.5) | Not examined | 10 (<2.5) |
| R3 | 3.8 (<1.3) | 15 (<0.8) | 52 (<0.8) | 2.5 (<0.8) |
| R4 | Damage | 4.3 (<1.4) | 1.4 (<1.4) | 41 (4.3) |
| R5 | <2.5 (<2.5) | 11 (<1.4) | <1.4 (<1.4) | 18 (1.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Urase, T.; Goto, S. Constituents of Coliform Species Contained in the Permeate of Microfiltration Membranes in Wastewater Treatment. Water 2024, 16, 1269. https://doi.org/10.3390/w16091269
Zhou S, Urase T, Goto S. Constituents of Coliform Species Contained in the Permeate of Microfiltration Membranes in Wastewater Treatment. Water. 2024; 16(9):1269. https://doi.org/10.3390/w16091269
Chicago/Turabian StyleZhou, Shuai, Taro Urase, and Saki Goto. 2024. "Constituents of Coliform Species Contained in the Permeate of Microfiltration Membranes in Wastewater Treatment" Water 16, no. 9: 1269. https://doi.org/10.3390/w16091269