Influence of High-Intensity Interval Training on Neuroplasticity Markers in Post-Stroke Patients: Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
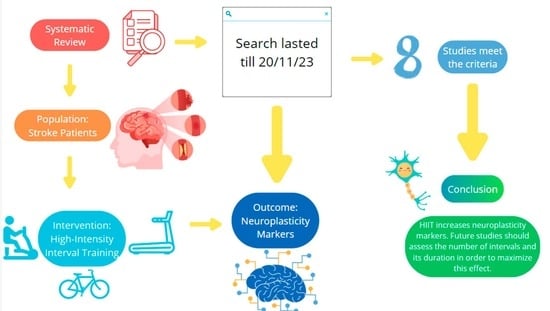
2.2. Article Search and Selection Process
2.3. Data Processing
- HIIT protocols
- ○
- Frequency of sessions
- ○
- Number of intervals
- ○
- Intensity
- Plasmatic neuroplasticity markers (such as BDNF, VEGF or lactate among others)
- Neurophysiological neuroplasticity markers (such as corticospinal excitability, cortical silent period or motor-evoked potentials)
- Other assessments (cognitive or quality of life scales)
- Demographic data (number of participants, gender and type of stroke)
2.4. Risk of Bias
3. Results
3.1. Study Characteristics
3.2. Frequency of Sessions
3.3. HIIT Intervals
3.4. Intensity Measures
3.5. Plasmatic Neuroplasticity Markers
3.6. Neurophysiological Neuroplasticity Markers
3.7. Other Measurements
3.8. Adverse Events
3.9. Risk of Bias
4. Discussion
Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Table Footnote Definitions
Appendix B. Search Strategy
- PubMed: Records identified—1007
- Strategy used (using MESH):
- (((((“chronic stroke” OR “Stroke”[Mesh]) AND “High-Intensity Interval Training”[Mesh] OR HIIT)
- AND “Neuronal Plasticity”[Mesh]) OR “Nerve Growth Factors”[Mesh]) OR “Nerve Growth
- Factor”[Mesh]) OR “Brain-Derived Neurotrophic Factor”[Mesh]) OR “Functional Magnetic
- Resonance Imaging” OR “Functional MRI”)
- Filters used: RCT, Last 5 years, Human.
- LILACS: Records identified—3
- Strategy used:
- stroke AND (“high-intensity interval training” OR HIIT) AND “neuronal plasticity”
- Filters used: RCT, Last 5 years,
- ProQuest: Records identified—156
- Strategy used:
- (“chronic stroke” OR stroke) AND (“high-intensity interval training” OR HIIT) AND (“neuronal
- plasticity” OR “Nerve growth factors” OR “Nerve growth factor” OR “Brain-derived
- neurotrophic factor” OR “Functinoal magnetic resonance imaging” OR “Functional MRI”)
- Filters used: Scientific magazine, Last 5 years, articles.
- PEDro: Records identified—11
- Strategy Used:
- “Stroke” AND “High-intensity interval training”
- Filters used: Articles from 01/01/2017 and RCT
- Web of Science (WOS): Records identified—6
- Strategy used:
- (“chronic stroke” OR stroke) AND (“high-intensity interval training” OR HIIT) AND (“neuronal
- plasticity” OR “Nerve growth factors” OR “Nerve growth factor” OR “Brain-derived
- neurotrophic factor” OR “Functinoal magnetic resonance imaging” OR “Functional MRI”)
- Filters used: Years 2017–2022, articles.
References
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An Updated Definition of Stroke for the 21st Century: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J.; Czajka, A.; Zielińska-Turek, J.; Jaroszyński, J.; Furtak-Niczyporuk, M.; Mela, A.; Poniatowski, Ł.A.; Drop, B.; Dorobek, M.; Barcikowska-Kotowicz, M.; et al. Brain Functional Reserve in the Context of Neuroplasticity after Stroke. Neural Plast. 2019, 2019, 9708905. [Google Scholar] [CrossRef]
- Heiss, W.-D.; Rosner, G. Functional Recovery of Cortical Neurons as Related to Degree and Duration of Ischemia. Ann. Neurol. 1983, 14, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Liu, R. Four Decades of Ischemic Penumbra and Its Implication for Ischemic Stroke. Transl. Stroke Res. 2021, 12, 937–945. [Google Scholar] [CrossRef]
- Castrén, E.; René, H. Neuronal Plasticity and Antidepressant Actions. Trends Neurosci. 2013, 36, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in Health, Resilience and Disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, J.M.; Krum, J.M.; Ruhrberg, C. VEGF in the Nervous System. Organogenesis 2010, 6, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Karantali, E.; Kazis, D.; Papavasileiou, V.; Prevezianou, A.; Chatzikonstantinou, S.; Petridis, F.; McKenna, J.; Luca, A.C.; Trus, C.; Ciobica, A.; et al. Serum Bdnf Levels in Acute Stroke: A Systematic Review and Meta-Analysis. Medicina 2021, 57, 297. [Google Scholar] [CrossRef]
- Bao, M.H.; Zhu, S.Z.; Gao, X.Z.; Sun, H.S.; Feng, Z.P. Meta-Analysis on the Association between Brain-Derived Neurotrophic Factor Polymorphism Rs6265 and Ischemic Stroke, Poststroke Depression. J. Stroke Cerebrovasc. Dis. 2018, 27, 1599–1608. [Google Scholar] [CrossRef]
- Stanne, T.M.; Aberg, N.D.; Nilsson, S.; Jood, K.; Blomstrand, C.; Andreasson, U.; Blennow, K.; Zetterberg, H.; Isgaard, J.; Svensson, J.; et al. Low Circulating Acute Brain-Derived Neurotrophic Factor Levels Are Associated with Poor Long-Term Functional Outcome after Ischemic Stroke. Stroke 2016, 47, 1943–1945. [Google Scholar] [CrossRef]
- Galvan, V.; Greenberg, D.; Jin, K. The Role of Vascular Endothelial Growth Factor in Neurogenesis in Adult Brain. Mini Rev. Med. Chem. 2006, 6, 667–669. [Google Scholar] [CrossRef]
- Kanazawa, M.; Takahashi, T.; Ishikawa, M.; Onodera, O.; Shimohata, T.; del Zoppo, G.J. Angiogenesis in the Ischemic Core: A Potential Treatment Target? J. Cereb. Blood Flow Metab. 2019, 39, 753. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Ninomiya, I.; Kanazawa, M. Angiogenesis and Neuronal Remodeling after Ischemic Stroke. Neural Regen. Res. 2020, 15, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Proia, P.; di Liegro, C.M.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a Metabolite and a Regulator in the Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef]
- Licht, T.; Rothe, G.; Kreisel, T.; Wolf, B.; Benny, O.; Rooney, A.G.; Ffrench-Constant, C.; Enikolopov, G.; Keshet, E. VEGF Preconditioning Leads to Stem Cell Remodeling and Attenuates Age-Related Decay of Adult Hippocampal Neurogenesis. Proc. Natl. Acad. Sci. USA 2016, 113, E7828–E7836. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Pan, C.Y.; Tseng, Y.T.; Chen, F.C.; Chang, Y.C.; Wang, T.C. Acute Effects of High-Intensity Interval Training and Moderate-Intensity Continuous Exercise on BDNF and Irisin Levels and Neurocognitive Performance in Late Middle-Aged and Older Adults. Behav. Brain Res. 2021, 413, 113472. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Boyne, P.; Meyrose, C.; Westover, J.; Whitesel, D.; Hatter, K.; Reisman, D.S.; Cunningham, D.; Carl, D.; Jansen, C.; Khoury, J.C.; et al. Exercise Intensity Affects Acute Neurotrophic and Neurophysiological Responses Poststroke. J. Appl. Physiol. 2019, 126, 431–443. [Google Scholar] [CrossRef]
- Boyne, P.; Meyrose, C.; Westover, J.; Whitesel, D.; Hatter, K.; Reisman, D.S.; Carl, D.; Khoury, J.C.; Gerson, M.; Kissela, B.; et al. Effects of Exercise Intensity on Acute Circulating Molecular Responses Poststroke. Neurorehabil. Neural Repair 2020, 34, 222–234. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nepveu, J.F.; Thiel, A.; Tang, A.; Fung, J.; Lundbye-Jensen, J.; Boyd, L.A.; Roig, M. A Single Bout of High-Intensity Interval Training Improves Motor Skill Retention in Individuals with Stroke. Neurorehabil. Neural Repair 2017, 31, 726–735. [Google Scholar] [CrossRef]
- Krawcyk, R.S.; Vinther, A.; Petersen, N.C.; Faber, J.; Iversen, H.K.; Christensen, T.; Lambertsen, K.L.; Rehman, S.; Klausen, T.W.; Rostrup, E.; et al. Effect of Home-Based High-Intensity Interval Training in Patients with Lacunar Stroke: A Randomized Controlled Trial. Front. Neurol. 2019, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Fu, T.C.; Huang, S.C.; Chen, C.P.C.; Wang, J.S. Increased Serum Brain-Derived Neurotrophic Factor with High-Intensity Interval Training in Stroke Patients: A Randomized Controlled Trial. Ann. Phys. Rehabil. Med. 2021, 64, 101385. [Google Scholar] [CrossRef] [PubMed]
- Abraha, B.; Chaves, A.R.; Kelly, L.P.; Wallack, E.M.; Wadden, K.P.; McCarthy, J.; Ploughman, M. A Bout of High Intensity Interval Training Lengthened Nerve Conduction Latency to the Non-Exercised Affected Limb in Chronic Stroke. Front. Physiol. 2018, 9, 377410. [Google Scholar] [CrossRef]
- Boyne, P.; Billinger, S.A.; Reisman, D.S.; Awosika, O.O.; Buckley, S.; Burson, J.; Carl, D.; DeLange, M.; Doren, S.; Earnest, M.; et al. Optimal Intensity and Duration of Walking Rehabilitation in Patients with Chronic Stroke. JAMA Neurol. 2023, 0394, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Valkenborghs, S.R.; van Vliet, P.; Nilsson, M.; Zalewska, K.; Visser, M.M.; Erickson, K.I.; Callister, R. Aerobic Exercise and Consecutive Task-Specific Training (AExaCTT) for Upper Limb Recovery after Stroke: A Randomized Controlled Pilot Study. Physiother. Res. Int. 2019, 24, e1775. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Dyar, K.A.; Treebak, J.T.; Jepsen, S.L.; Ehrlich, A.M.; Ashcroft, S.P.; Trost, K.; Kunzke, T.; Prade, V.M.; Small, L.; et al. Atlas of Exercise Metabolism Reveals Time-Dependent Signatures of Metabolic Homeostasis. Cell Metab. 2022, 34, 329–345.e8. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Timmons, J.A.; Baar, K.; Davidsen, P.K.; Atherton, P.J. Is Irisin a Human Exercise Gene? Nature 2012, 488, E9–E10. [Google Scholar] [CrossRef] [PubMed]
- Shahroudi, A.; Moradi-kor, N. The Effect of Exercise and Drugs on Cognitive Function and BDNF Protein. IIOAB J. 2016, 7, 87–96. [Google Scholar]
- Vital, T.M.; Stein, A.M.; Coelho, F.G.d.M.; Arantes, F.J.; Teodorov, E.; Santos-Galduróz, R.F. Physical Exercise and Vascular Endothelial Growth Factor (VEGF) in Elderly: A Systematic Review. Arch. Gerontol. Geriatr. 2014, 59, 234–239. [Google Scholar] [CrossRef] [PubMed]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A.M.; Andrushko, J.W.; Della Gatta, P.A.; Teo, W.-P. Acute Effects of High-Intensity Aerobic Exercise on Motor Cortical Excitability and Inhibition in Sedentary Adults. Front. Psychol. 2022, 13, 814633. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Utesch, T.; Wu, J.; Robertson, S.; Liu, J.; Hu, G.; Chen, H. Effects of Different Protocols of High Intensity Interval Training for VO2max Improvements in Adults: A Meta-Analysis of Randomised Controlled Trials. J. Sci. Med. Sport 2019, 22, 941–947. [Google Scholar] [CrossRef]
- Krawcyk, R.S.; Vinther, A.; Petersen, N.C.; Faber, J.; Iversen, H.K.; Christensen, T.; Klausen, T.W.; Kruuse, C. High-Intensity Training in Patients with Lacunar Stroke: A One-Year Follow-Up. J. Stroke Cerebrovasc. Dis. 2023, 32, 106973. [Google Scholar] [CrossRef]
- Mann, T.; Lamberts, R.P.; Lambert, M.I. Methods of Prescribing Relative Exercise Intensity: Physiological and Practical Considerations. Sports Med. 2013, 43, 613–625. [Google Scholar] [CrossRef]
- Hsieh, S.S.; Chueh, T.Y.; Huang, C.J.; Kao, S.C.; Hillman, C.H.; Chang, Y.K.; Hung, T.M. Systematic Review of the Acute and Chronic Effects of High-Intensity Interval Training on Executive Function across the Lifespan. J. Sports Sci. 2021, 39, 10–22. [Google Scholar] [CrossRef]
- Marquez, C.M.S.; Vanaudenaerde, B.; Troosters, T.; Wenderoth, N. High-Intensity Interval Training Evokes Larger Serum BDNF Levels Compared with Intense Continuous Exercise. J. Appl. Physiol. 2015, 119, 1363–1373. [Google Scholar] [CrossRef]
- Walsh, J.J.; Tschakovsky, M.E. Exercise and Circulating BDNF: Mechanisms of Release and Implications for the Design of Exercise Interventions. Appl. Physiol. Nutr. Metab. 2018, 43, 1095–1104. [Google Scholar] [CrossRef]
- Della-Maggiore, V.; Villalta, J.I.; Kovacevic, N.; McIntosh, A.R. Functional Evidence for Memory Stabilization in Sensorimotor Adaptation: A 24-h Resting-State Fmri Study. Cereb. Cortex 2017, 27, 1748–1757. [Google Scholar] [CrossRef]
- Sathyamurthy, A.; Barik, A.; Dobrott, C.I.; Matson, K.J.E.; Stoica, S.; Pursley, R.; Chesler, A.T.; Levine, A.J. Cerebellospinal Neurons Regulate Motor Performance and Motor Learning. Cell Rep. 2020, 31, 107595. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, C.C.; Alcantara, C.C.; French, M.A.; Li, X.; Matt, K.S.; Kim, H.E.; Morton, S.M.; Reisman, D.S. A Single Exercise Bout and Locomotor Learning after Stroke: Physiological, Behavioural, and Computational Outcomes. J. Physiol. 2018, 596, 1999–2016. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, C.C.; Helm, E.E.; La, K.A.; Morton, S.M.; Reisman, D.S. The Feasibility of an Acute High-Intensity Exercise Bout to Promote Locomotor Learning after Stroke. Top Stroke Rehabil. 2019, 25, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Limaye, N.S.; Carvalho, L.B.; Kramer, S. Effects of Aerobic Exercise on Serum Biomarkers of Neuroplasticity and Brain Repair in Stroke: A Systematic Review. Arch. Phys. Med. Rehabil. 2021, 102, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Gripp, F.; Nava, R.C.; Cassilhas, R.C.; Esteves, E.A.; Magalhães, C.O.D.; Dias-Peixoto, M.F.; de Castro Magalhães, F.; Amorim, F.T. HIIT Is Superior than MICT on Cardiometabolic Health during Training and Detraining. Eur. J. Appl. Physiol. 2021, 121, 159–172. [Google Scholar] [CrossRef]
- Ashcroft, S.K.; Ironside, D.D.; Johnson, L.; Kuys, S.S.; Thompson-Butel, A.G. Effect of Exercise on Brain-Derived Neurotrophic Factor in Stroke Survivors: A Systematic Review and Meta-Analysis. Stroke 2022, 53, 3706–3716. [Google Scholar] [CrossRef]
- Boyne, P.; Miller, A.; Schwab, S.M.; Sucharew, H.; Carl, D.; Billinger, S.A.; Reisman, D.S. Training Parameters and Longitudinal Adaptations That Most Strongly Mediate Walking Capacity Gains from High-Intensity Interval Training Post-Stroke. medRxiv 2023, 7499, 2023-02. [Google Scholar]
- French, M.A.; Morton, S.M.; Pohlig, R.T.; Darcy, S. Reisman The Relationship between BDNF Val66Met Polymorphism and Functional Mobility in Chronic Stroke Survivors. Top. Stroke Rehabil. 2018, 25, 276–280. [Google Scholar] [CrossRef]
- Liu, X.; Fang, J.C.; Zhi, X.Y.; Yan, Q.Y.; Zhu, H.; Xie, J. The Influence of Val66Met Polymorphism in Brain-Derived Neurotrophic Factor on Stroke Recovery Outcome: A Systematic Review and Meta-Analysis. Neurorehabil. Neural Repair 2021, 35, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, G.; Pellegrino, G.; Capone, F.; Assenza, G.; Florio, L.U.C.I.A.; Falato, E.M.M.A.; Lotti, F.; Di Lazzaro, V. Val66Met BDNF Polymorphism Implies a Different Way to Recover From Stroke Rather Than a Worse Overall Recoverability. Neurorehabil. Neural Repair 2016, 30, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.J.; Zhang, J.J. Changes in HIF-1α, VEGF, NGF and BDNF Levels in Cerebrospinal Fluid and Their Relationship with Cognitive Impairment in Patients with Cerebral Infarction. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2013, 33, 433–437. [Google Scholar] [CrossRef]
- Niwa, A.; Nishibori, M.; Hamasaki, S.; Kobori, T.; Liu, K.; Wake, H.; Mori, S.; Yoshino, T.; Takahashi, H. Voluntary Exercise Induces Neurogenesis in the Hypothalamus and Ependymal Lining of the Third Ventricle. Brain Struct. Funct. 2016, 221, 1653–1666. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, R.; Cariati, I.; Tarantino, U.; D’arcangelo, G.; Tancredi, V. Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases. J. Funct. Morphol. Kinesiol. 2022, 7, 38. [Google Scholar] [CrossRef]
- Le Blanc, J.; Fleury, S.; Boukhatem, I.; Bélanger, J.C.; Welman, M.; Lordkipanidzé, M. Platelets Selectively Regulate the Release of BDNF, But Not That of Its Precursor Protein, ProBDNF. Front. Immunol. 2020, 11, 575607. [Google Scholar] [CrossRef]
- Lüscher, C.; Nicoll, R.A.; Malenka, R.C.; Muller, D. Synaptic Plasticity and Dynamic Modulation of the Postsynaptic Membrane The Biochemical Composition of the Postsynaptic Membrane and the Structure of Dendritic Spines May Be Rapidly Modulated by Synaptic Activity. Here We Review These Findings, Discuss Th. Nat. Rev. Neurosci. 2000, 3, 545–550. [Google Scholar] [CrossRef]
- Ito, U.; Kuroiwa, T.; Nagasao, J.; Kawakami, E.; Oyanagi, K. Temporal Profiles of Axon Terminals, Synapses and Spines in the Ischemic Penumbra of the Cerebral Cortex: Ultrastructure of Neuronal Remodeling. Stroke 2006, 37, 2134–2139. [Google Scholar] [CrossRef]


| Authors | Population | Interventions | HIIT | Measures | Outcomes |
|---|---|---|---|---|---|
| Nepveu et al., 2017 [23] | n = 22; Time after stroke: >6 months. | Motor task for skill retention (time on target); Rest (Group 1); HIIT (1 session) (Group 2); Motor task for skill retention (time on target). | 2 min warm-up (25% peak workload GTX), 3 × 3 min (100% peak workload GTX) with active recovery of 2 min (25% peak workload GTX). | GTX, VO2, HRmax, Pemax, CSE, SICI, ICF, CSP. | HIIT increases skill retention. |
| Krawcyk et al., 2019 [24] | n = 63; Time after stroke: 3 weeks after episode; | Usual care (medication and lifestyle advice); HIIT (5 days/week, 12 weeks). | 3 × 3 min with 2 min of active recovery, 77–93% of the maximum heart rate. | Pro-ADM, Pro-ANP, copeptin, IL-6, TNF, ICAM-1, VCAM-1, VEGF, E-selectin. | Ambiguous response of biomarkers, W of bicycle did not increase. |
| Boyne et al., 2019 [19] | n = 16; Time after stroke: >6 months. | Treadmill-HIIT; Seated-stepper-HIIT; Seated-stepper-HIIT (revised); Treadmill-MICT (3 sessions); Crossover randomized clinical trial. | Treadmill 20 min of exercise, repeated 30 s bursts at maximum tolerated speed (0% incline). Recovery from 60 to 30 s after 5 min. Seated stepper high-HIIT—same burst and recovery durations as HIIT-treadmill. Bursts at maximum possible cadence against 50% of maximal resistance. Revised HIIT stepper: No resistance, mean intensity target 70–85% HRR. MCT-treadmill walking with aerobic intensity of 45 5% HRR. | Blood lactate, VO2, HRR, CSA CSP, BDNF. | HIIT associated with increases in BDNF, lactate and VO2. BDNF not associated with motor activation threshold or CSP. |
| Boyne et al., 2020 [20] | n = 16; Time after stroke: >6 months. | Treadmill-HIIT; Seated-stepper-HIIT; Seated-stepper-HIIT (Modified); Treadmill-MICT (3 sessions); Crossover randomized clinical trial. | Same protocol as stated above. | Blood lactate, VEGF, IGF-1, BDNF. | Treadmill HIIT increases VEGF, BDNF and IGF-1. High lactate levels associated to a higher number of neural markers. |
| Hsu et al., 2021 [25] | n = 23; Time after stroke: >3 months. | MICT; HIIT (36 sessions). | 3 min warm-up 30% VO2 peak, 5 × 3 min intervals 80% VO2 peak, 3 min cooldown 30% VO2 peak, | BDNF, neurite growth (%), cerebral tissue. Hb: total hemoglobin, oxyhemoglobin, deoxyhemoglobin. | Increased cerebral blood flow and O2. BDNF increase can result in neural growth. |
| Abraha et al., 2018 [26] | n = 12; Time after stroke: >6 months. | MICT; HIIT. | 5 min warm-up increasing workload till 80%VO2 peak, 5 × 2 min intervals at 80% VO2 peak, 5 × 2 min active recovery at 40% VO2 peak. | VO2 peak, HR, MEP, MEP latency. | HIIT lengthened nerve conduction latency. This effect was intensity-dependent. |
| Boyne et al., 2023 [27] | n = 55; Time after stroke: >6 months; Gender: M(36) F(19); Type of stroke: I(34) H(21). | MICT; HIIT. | Treadmill 20 min of exercise, repeated 30 s bursts at maximum tolerated speed (0% incline), recovery from 60 to 30 s after 5 min. | Blood lactate, VO2, 6-MWT. | Higher intensity seems better than moderate intensity for improving walking capacity. |
| Valkenborghs et al., 2019 [28] | n = 20; Time after stroke: >6 months; Gender: M(11) F(9); Type of stroke: I/H (20). | AEX + TST; TST. | 4 × 4 min interval, 85%Hrmax, 3 × 3 min active recovery, 70%Hrmax, 5 min light-to-moderate intensity (cooldown). | BDNF, ARAT, WMFT HRmax, VO2max. | Both groups improved their performance in daily activities with HIIT improving retention nearly twice as much as TST alone. However, BDNF decreased in both groups. |
| Study | Random Allocation | Concealed Allocation | Groups Similar at Baseline | Participant Blinding | Therapist Blinding | Assessor Blinding | <15% Dropouts | Intention to Treat Analysis | Between Group Difference Reported | Point Estimate and Variability Reported | Total (0 to 10) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nepveu et al., 2017 [23] |  |  |  |  |  |  |  |  |  |  | 5 |
| Krawcyk et al., 2019 [24] |  |  |  |  |  |  |  |  |  |  | 7 |
| Boyne et al., 2019 [19] |  |  |  |  |  |  |  |  |  |  | 5 |
| Boyne et al., 2020 [20] |  |  |  |  |  |  |  |  |  |  | 5 |
| Hsu et al., 2021 [25] |  |  |  |  |  |  |  |  |  |  | 6 |
| Abraha et al., 2018 [26] |  |  |  |  |  |  |  |  |  |  | 5 |
| Boyne et al., 2023 [27] |  |  |  |  |  |  |  |  |  |  | 8 |
| Valkenborghs et al., 2019 [28] |  |  |  |  |  |  |  |  |  |  | 8 |
 = Does not meet the criteria
= Does not meet the criteria  = Does meet the criteria.
= Does meet the criteria.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero-Almagro, G.; Bernal-Utrera, C.; Geribaldi-Doldán, N.; Nunez-Abades, P.; Castro, C.; Rodriguez-Blanco, C. Influence of High-Intensity Interval Training on Neuroplasticity Markers in Post-Stroke Patients: Systematic Review. J. Clin. Med. 2024, 13, 1985. https://doi.org/10.3390/jcm13071985
Montero-Almagro G, Bernal-Utrera C, Geribaldi-Doldán N, Nunez-Abades P, Castro C, Rodriguez-Blanco C. Influence of High-Intensity Interval Training on Neuroplasticity Markers in Post-Stroke Patients: Systematic Review. Journal of Clinical Medicine. 2024; 13(7):1985. https://doi.org/10.3390/jcm13071985
Chicago/Turabian StyleMontero-Almagro, Gines, Carlos Bernal-Utrera, Noelia Geribaldi-Doldán, Pedro Nunez-Abades, Carmen Castro, and Cleofas Rodriguez-Blanco. 2024. "Influence of High-Intensity Interval Training on Neuroplasticity Markers in Post-Stroke Patients: Systematic Review" Journal of Clinical Medicine 13, no. 7: 1985. https://doi.org/10.3390/jcm13071985