3.1. Phase 1—Influence of Core/Shell Ratio on Microcapsule Morphology and Reaction Percent Yield
The optimal core-to-shell ratio for the emulsification and encapsulation of TEGDMA in PUF shells was investigated and the results are shown in
Figure 2. The percentage yield of the reactions ranged from 44.9% to 9.1%, with the highest results observed for the core/shell ratio of 6/1 and the lowest at 9/1 (
Figure 3A). The performance of the microencapsulation reaction based on an oil-in-water emulsion is highly dependent on several factors, including the equilibrium between the inner oily and outer water phases. This equilibrium hinges on differences in hydrophilicity/hydrophobicity between the phases, emulsification stability, viscosity, the solubility of the shell precursor compounds in water, and reaction kinetics [
15,
16]. In summary, under the influence of high shear stresses produced by the agitation, the formation of microdroplets during emulsification relies on the dispersion of TEGDMA into the water phase, resulting in droplets stabilized by the presence of the surfactant pEMA. These emulsion droplets undergo spherical-to-ellipsoidal shape deformation and subsequent breakup into smaller drops. This process is highly dependent on the media’s viscosity, shear rate, and interfacial tension [
17]. An intricate interplay exists between the media’s viscosity and the concentration of surfactant. An increase in the volume of TEGDMA in the core/shell ratios of 7.5/1 to 9/1 may have significantly increased the viscosity of the emulsion. This increased viscosity may render the oily phase more resistant to the shear stress transmitted by the water phase due to agitation. In simpler terms, since the agitation rate was held constant for all the groups, emulsions with a higher viscosity experienced a lower magnitude of stress transmitted by the water phase, leading to reduced droplet formation. Moreover, the droplet coalescence and collision in these systems are favored due to the reduced distance between them caused by the increased concentration of cargo in the oil phase [
15]. Additionally, surfactants are added to microemulsions to lower interfacial tension and, consequently, the internal pressure of microdroplets [
18]. This reduction in tension is essential for microdroplet formation and preventing their coalescence. In these groups, the lower overall concentration of surfactant, due to the increased volume of TEGDMA, may not have been sufficient to migrate toward the interface within the interfacial film, lower the stress, facilitate microdroplet formation, and prevent coalescence. As a result, there was a significantly decreased reaction yield and an agglomeration of the shell polymer precursors in the water phase, forming sheet-like networks (
Figure 3B) [
18]. Lastly, limited shell polymer precursors may also impose a limitation to completely cover the healing microdroplets and often result in the formulation of thin and fragile shells that are susceptible to the shear forces during the synthesis.
Interestingly, core/shell ratios groups 3/1 and 4.5/1 resulted in larger microcapsules (104.5 ± 63.5 µm and 131.2 ± 97.3 µm, respectively) than groups 7.5/1 and 9/1 (62.3 ± 30.7 µm and 79.4 ± 61.4 µm, respectively). It is related to the fact that, at higher ratios such as those induced in groups 3/1 and 4.5/1, the surfactant molecules tend to orient themselves substantially parallel to each other. This behavior differs from their usual arrangement, where they straddle the interface with the polar group in the water phase and the hydrophobic tail in the cargo phase. This change affects the stability of the emulsion and the breakup of droplets, leading to the formation of larger droplets and, ultimately, larger microcapsules [
19]. Regarding the reaction yields, 3/1 and 4.5/1 resulted in values as low as the groups 7.5/1 and 9/1. The low yield may be related to an extensive degree of demulsification, induced by the destabilization of the emulsion and the subsequent separation of oil and water phases [
19,
20]. At low concentrations in a system, the surfactant can adsorb onto the surfaces or interfaces and significantly alter their surface or interfacial free energies, as discussed previously. In addition, the increased water content leads to the external pressure of the water phase becoming lower than the internal pressure, which is translated into an increase in the interface film thinning and, consequently, the increased water droplet drawing and demulsification [
19].
Therefore, for the system tested in this study, the core/shell ratio of 6/1 resulted in a more stable equilibrium between the oil and water phases among the tested concentrations. This ratio was selected for the next phases of the study.
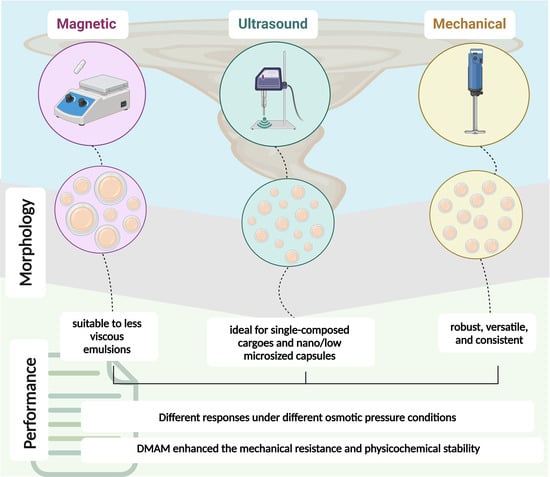
3.2. Phase 2—Influence of Microemulsification Technique on Microcapsule Morphology and Reaction Percent Yield
Different techniques can be employed to promote the emulsification of a two-phase reaction. In this study, magnetic stirring (MA), ultrasonication (U), and mechanical stirring (ME) were compared. These techniques were employed to encapsulate two distinct cargo systems: the first composed of 100 wt.% TEGDMA, and the second composed of 80 wt.% TEGDMA + 20 wt.% DMAM (57 mol.% TEGDMA + 43 mol.% DMAM). DMAM is a low-molecular-weight tertiary acrylamide (~99.3 g/mol) and is consequently highly hydrophilic (logP = 0.3) [
21]. The morphological characteristics of microcapsules, the size distribution, the averages of reaction percent yield (%), microcapsule diameter (µm), and the encapsulation efficiency (%) results are presented in
Figure 4.
Ensuring that the diameter of the PUF microcapsules meets the requirements for practical applications is crucial. Excessively small capsules may contain a limited amount of cargo, while overly large capsules may lack stability, become fragile, and fail to resist handling. In general, the microemulsification technique played a pivotal role, with groups synthesized through mechanical stirring exhibiting a more uniform size distribution, as highlighted by the narrower profiles depicted in the histograms in
Figure 4A. The capsule sizes for the 100T-ME group ranged from 50 to 225 µm (average size = 142.9 ± 12.1 µm), while for the 80T/20D-ME group, the range was from 25 to 200 µm (average size = 100.3 ± 16.3 µm). It is related to the fact that the shear stress generated by the impeller blades of mechanical stirrers is distributed more evenly throughout the solution, resulting in microdroplets with more consistent sizes and, consequently, a more uniform size distribution of microcapsule diameters [
22]. On the other hand, groups synthesized using magnetic stirring yielded microcapsules with a broader size distribution. Capsule sizes for the 100T-MA group ranged from 50 to 550 µm (average size = 160.5 ± 88.0 µm), and for the 80T/20D-MA group, they spanned from 50 to 450 µm (average size = 158.9 ± 24.0 µm). In general, the magnitude of the shear forces generated by magnetic stirrers is highly heterogeneously distributed, with high intensity near the stir bar and becoming almost negligible towards the periphery of the reaction vial [
23]. The scenario is even more critical when the viscosity of the emulsion is high because it imposes more resistance to the shear forces propagation. Therefore, magnetic stirring seems to be appropriate for emulsifying low-to-medium-viscosity solutions. In contrast, solutions with higher viscosity benefit from mechanical stirrers equipped with impellers due to the greater magnitude of the shear stress generated by the blades in comparison with magnetic stir bars [
24].
While the composition of the cargo did not play a significant role in the microcapsule sizes of the mechanical and magnetic emulsification techniques, it had a substantial impact on ultrasonication. The 100T-U group exhibited a capsule size distribution ranging from 4 to 14 µm (average size = 6.5 ± 0.6 µm), whereas the 80T/20D group displayed a broader distribution, spanning from 15 to 135 µm (average size = 56.1 ± 8.05 µm) (
Figure 4A). In ultrasonication, the horn-tip produces vibrations that generate ultrasonic waves. These waves lead to the formation of cavitation bubbles, which are ruptured upon contact with the dispersed phase [
25]. This rupture process results in the formation of emulsions with smaller microdroplets compared to other methods, making it highly suitable for therapeutic applications that require low micro- or nano-sized capsules. The differences between the 100T-U and 80T/20D-U groups may be attributed to several challenges associated with the ultrasonication method. Firstly, an abundance of cavitation bubbles can lead to collisions among the bubbles, resulting in uneven bubble collapse, which affects the interaction between the bubbles and the cargo, thus disrupting the stability of microdroplets. Secondly, cavitation bubbles can accumulate around the ultrasonic probe, imposing a challenge for the uniform transmission of ultrasonic waves throughout the cargo [
26]. This situation often leads to the formation of smaller microdroplets near the ultrasonic probe, with sizes increasing as the distance from the probe increases, which results in non-homogeneous microdroplet sizes. In fact, it has been shown that formation and cavitation collapse are the major contributing factors to the size and polydispersity index, which are affected by the cavitation threshold of the emulsion, the location at which the ultrasonic horn is placed during the reaction, the acoustic pressure at the oil–water interface, and the acoustic pressure in the continuous phase [
27]. Since all the parameters were standardized and the only difference between the groups was the composition of the cargo, it is reasonable to hypothesize that the incorporation of DMAM (20 wt.% and 43 mol.%) increased the cavitation threshold of the emulsion due to the increased concentration of hydrogen bonding. The presence of strong hydrogen bonds creates strong intermolecular forces that hold the molecules of the emulsion together, which opposes the formation, propagation, and growth of the cavitation bubbles since additional energy is required to break these intermolecular forces [
28]. Furthermore, hydrogen bonds play a role in diminishing the stability of cavitation bubbles. These bubbles become more susceptible to collapse because hydrogen bonds can easily reform after the expansion of the bubble, resulting in either the shrinkage or rupture of the bubble [
28]. Therefore, DMAM-containing emulsions may have presented higher cavitation thresholds, leading to the formation of larger and more heterogenous emulsion droplets and, consequently, microcapsules [
27]. In addition, oil phases composed of more than one compound are more susceptible to the Ostwald ripening phenomenon, which leads to a shift toward a broader size distribution with a higher average droplet size [
29]. In summary, when the oil phase consists of two liquids, the more water-soluble compound can diffuse through the aqueous phase, leading to the coalescence of smaller droplets into larger ones [
15,
30]. This effect was more pronounced during ultrasonication in comparison to magnetic and mechanical stirring, possibly due to the generation of smaller microdroplets via this technique, as discussed earlier. Smaller droplets exhibit a higher curvature, resulting in increased Laplace pressure and, consequently, facilitating the migration of the cargo from smaller to larger droplets [
30].
It is important to note that the clear circles in the core of the microcapsules observed in the optical micrographs (
Figure 4A) are air microbubbles trapped within the cargo. The entrapment of air bubbles into the core of the microcapsules results from air being incorporated during the agitation process required to form the emulsion in the double-phase encapsulation technique [
31]. The visibility of these bubbles varies depending on the focus on the core or shell during optical microscopy imaging. Over time, these bubbles are released, and the wrinkles on the microcapsule shells become more evident [
31].
In terms of the physical aspect and morphological features, 100T-MA and 80T/20D-MA tended to form agglomerates, as evidenced via the SEM micrography (
Figure 4A). The agglomerates were even resistant to the optimized rinsing and filtration procedures. It is important to note that the rinsing protocol was optimized and tailored to the different cargo systems encapsulated in this study. Standard procedures described in the literature involve rinsing the microcapsules with acetone. However, the acetone-based rinsing method in this study proved harmful, inducing a dramatic plasticizing effect on the polymeric shells, and leading to the premature rupture of the microcapsules (
Figure 5). Although there are no specific studies that have investigated the impact of the acetone-based rinsing method on the physicochemical stability of the poly(urea-formaldehyde) networks, acetone is a well-known plasticizing agent that has the potential to cause the softening, smearing, or even dissolution of polymeric networks [
32]. In the context of PUF microcapsules, the plasticizing effects resulting from the exposure to acetone may have adversely affected the shell’s mechanical properties to a degree where the mild vacuum applied during filtration posed a more significant challenge than the polymeric network of the shells could withstand. Therefore, rinsing protocols with several different solvents were tested (data not presented here) and the integrity of the microcapsules was analyzed under optical microscopy after filtration. For the systems tested in this study, the optimal protocol consisted of the decantation of the microcapsules in distilled water at 10 times the volume of the reaction for 1 h, followed by rinsing with 200 mL of hexanes for 100T groups and 200 mL of hexanes + 100 mL of chloroform for 80T/20D groups. Decantation in water proved to be an effective strategy for physically separating the microcapsules, which tended to accumulate at the bottom of the vial, from the unreacted starting materials and solvents (
Figure 5). This significantly reduced the volume of solvents required for the rinsing phase. Hexane-based rinsing was the optimal solvent for 100T systems, being capable of dissolving the unreacted compounds without compromising the physical stability of the polymeric shells and maintaining the integrity of the microcapsules. For the 80T/20D systems, in addition to the hexanes, an extra rinse with chloroform was required. This is related to the fact that due to the low relative polarity, hexanes can dissolve the unencapsulated TEGDMA but not DMAM, which is a highly polar compound (
Figure 5).
Although the optimization and tailoring of the rinsing/filtration protocols resulted in a free-flowing, light, and dry appearance for most experimental groups, as mentioned earlier, the magnetic stirring groups, especially 100T-MA, exhibited an agglomerated and oily aspect, indicating cargo leakage. In addition, this group presented a significant amount of polymer sheets, which are essentially polymeric structures that did not transform into microcapsules or are residues of microcapsules ruptured during the synthesis. This may be due to the significantly larger microcapsule average size seen in this group in comparison to the other groups. Larger microcapsules are ruptured more easily than smaller microcapsules due to their lower stability, compromised shell stiffness, and greater hydrostatic pressure imposed by the higher volume of cargo [
33]. Moreover, larger capsules are more susceptible to settling at the bottom of the reaction flask, where they are subjected to intense mechanical stress. The strong magnetic field created by the proximity of the stirrer bar and the magnets on the plate generates stronger shear and compressive forces, due to collisions of the microcapsules with the stirrer bar or other microcapsules in the reaction mixture [
24]. These forces are particularly detrimental to the capsules because they are still undergoing thermopolymerization, which means that their shells have not reached the maximum crosslinking density yet and, consequently, present lower mechanical resistance to endure the mechanical challenge.
In addition, given the lower and heterogeneous gradient of the shear forces magnitude generated by the magnetic stirring method, as discussed earlier, it is expected that the shell precursors were not homogeneously distributed into the reaction media. This non-uniform distribution may have resulted in a heterogeneous PUF polymeric network and, therefore, suboptimal mechanical responses. In fact, even the microcapsules that survived into the synthesis process started showing some level of cargo leakage after rinsing and filtration, evidenced by the oily physical aspect and the formation of microcapsule agglomerates. It may indicate that the high level of heterogeneity of the poly(urea-formaldehyde) network formed during the magnetic stirring. It is important to highlight that highly crosslinked polymeric networks, such as poly(urea-formaldehyde), are subject to experience heterogeneity due to several factors such as the randomness of chain compositions, the polydispersity of the polymer chains between reticulation nodes, the uneven distribution of the chemical crosslinkers leading to non-uniform reticulation nodes, random network topologies, and the lack of uniformity of the reticulation node density [
34]. As heterogeneities are progressively introduced into these highly crosslinked networks, a dramatic impact on their topological structures and mechanical properties is observed [
34]. Therefore, it seems that the high disparity in the shear forces distribution in the magnetic stirring contributed to the formation of a highly porous and stiff poly(urea-formaldehyde) network that allowed for the leakage of the cargo and eventual rupture of the microcapsule.
Interestingly, the 80T/20D-MA group exhibited better performance than the 100T-MA group, indicating that the composition of the cargo plays a crucial role in the stability and integrity of the microcapsules. The key role played by the cargo composition was also evidenced by the physical and morphological differences between the 100T-U and 80T/20D-U groups. While the 100T-U microcapsules were highly free-flowing, lightweight, and non-sticky, the 80T/20D-U capsules were sticky and exhibited some level of cargo leakage after drying (
Figure 4A). As mentioned earlier, the introduction of a more hydrophilic compound into the cargo system is particularly challenging in ultrasonication oil-in-water emulsion. This challenge arises due to the magnification of the Ostwald ripening phenomenon, leading to a dramatic increase in the overall diameter of the microcapsules from 6.5 μm to 56.1 μm, and resulting in lower microcapsule stability.
However, a noticeable impact on the cargo composition was not observed when mechanical stirring was used as the microemulsification technique. This observation is attributed to the morphological similarities found between the 100T-ME and 80T/20D-ME microcapsules. In fact, the robustness of the mechanical stirring technique regardless of the cargo systems tested in this study is also reflected in the highest values of the reaction percent yield exhibited by 100T-ME and 80T/20D-ME, ranging from 69.9 ± 9.9% to 60.3 ± 1.7%, respectively (
Figure 4B). The ultrasonication groups showed over 50% lower reaction percent yield compared to mechanical stirring, with values ranging from 30.7 ± 9.3% to 30.4 ± 1.94% for the 100T-U and 80T/20D-U groups, respectively (
Figure 4B). For the 80T/20D-U group, it may be mainly related to some loss of DMAM as it may have diffused through the aqueous phase due to Ostwald ripening [
35]. However, DMAM only accounts for 20 wt.% of the cargo composition, and even if all the DMAM was lost, it would not explain the 50% reduction in yield shown by this group. The explanation may rely on the impact on the core/shell ratio due to the reduction in the oil phase and the increase in the aqueous phase. If 25 wt.% of the DMAM was lost from the oil phase, the reaction ended up with a core/shell ratio of 4.5/1 instead of the optimal 6/1. As shown in the first phase of this study, this increase in the core/shell ratio impacts the equilibrium of the emulsion and leads to suboptimal reaction yield. However, Interestingly, 100T-U showed a similar yield to 80T/20D-U; therefore, DMAM loss is not the only reason for the lower percent yield in ultrasonication groups. It seems that TEGDMA, although more hydrophobic than DMAM, also lost some mass to the aqueous phase during ultrasonication. This may happen only in this technique because the droplets formed are at least one order of magnitude smaller than the ones formed in mechanical and magnetic stirring techniques, contributing to their coalescence and some loss of the cargo. Since the loss of TEGDMA may have increased the viscosity of the water phase, it may have reduced cavitation and, ultimately, the reaction yield [
26]. While the microemulsification technique played a key role in the reaction percent yield, the differences in the cargo systems did not affect it significantly (
Figure 4B). It is well known that the equilibrium between the oil and aqueous phases is a pivotal factor in determining the reaction yield of oil-in-water double emulsion reactions. Importantly, the replacement of 20 wt.% TEGDMA with DMAM seems not to have compromised this equilibrium. In terms of cargo encapsulation efficiency, the values ranged from 97.9 ± 0.4% to 90.5 ± 4.4%, with the lowest values presented by the ultrasonication groups (
Figure 4B). This was anticipated, given the significantly smaller size of 100T-U and 80T/20D-U, implying a diminished capacity for carrying cargo. Nevertheless, it is crucial to highlight that achieving a 90% encapsulation efficiency is a remarkable outcome, indicating success in optimizing the microencapsulation reactions.
3.3. Phase 3—Physicochemical Stability of the Microcapsules under Different Osmotic Pressures
In the last phase of this study, the microcapsules synthesized in phase 2 were stored dry at 4 °C, in distilled water, or in 0.1 M of phosphate-buffered saline (PBS) for 24 h, 1 week, 2 weeks, or 4 weeks. The morphological changes in the microcapsules were monitored using optical microscopy, and the cargo volume variation was quantified using the acetone extraction technique. The objectives were to (1) identify the optimal conditions for the storage of the microcapsules to maximize their integrity; (2) understand how changes in the core composition impact the microcapsule responses; (3) probe the differences in microcapsules mechanical properties due to different microemulsification techniques; and (4) gain insight into how the versatile PUF microcapsules can have their properties tailored for different purposes and therapeutic niches.
The results for 100T groups are depicted in
Figure 6,
Figure 7 and
Figure 8. In general, the microcapsules stored dry at 4 °C for 24 h, 1 week, and 2 weeks did not see a significant change in integrity and loss of cargo regardless of the microemulsification technique. These capsules showed the highest degrees of wrinkles on their shells and structural deformation from spherical round-shaped to a flattened oval format. After 4 weeks, there was a slight increase in the cargo loss that, although it was not statistically significant, led to microcapsules clumping, mainly for the 100T-MA group. It was anticipated that dry storage at low temperatures would result in the most stable behavior, as the capsules are subjected to much milder conditions. This expectation is based on the fact that, in a dry environment, morphological changes are mainly associated with the mechanical properties of the polymeric shell given the low hydrostatic and external pressures imposed by the liquid core and the environment, respectively [
36]. In other words, the stability of the microcapsules will be mainly related to shell thickness, the polymeric features of the poly(urea-formaldehyde) networks, and the mechanical properties of the shells [
37].
The flattening and wrinkling observed in these microcapsules seem to be mainly associated with the incomplete filling of the shells with core material or the loss of some core material over time [
38]. Given the fact that the microcapsules did not form agglomerates or present an oily surface, it may mean that some residual solvent incorporated into the core during synthesis or rinsing and filtration procedures further evaporated over the storage time. Interestingly, the 100T-MA group started clumping, and some free cargo marked in orange was identified in the vial after 4 weeks, indicating core leaking. This observation corroborates with the suboptimal performance presented by this group in phase 2, which was mainly attributed to the low mechanical properties of their poly(urea-formaldehyde) network shells and the larger size of the microcapsules, making them more fragile. Although the magnitude of hydrostatic pressure imposed by the liquid core expected in microsystems is low, it seems that in cases where the shell properties are suboptimal, this pressure overwhelms the polymer resistance over time, making it incapable of maintaining a resistant physical barrier to keep the cargo restrained within the microcapsule core.
On the other hand, noticeable structural changes were seen in 100T microcapsules incubated in water and PBS. In water, the microcapsules showed significant swelling that led to a diameter increase at approximately 51%, 30%, and 46% for the magnetic, ultrasonication, and mechanical stirring groups, respectively. This was expected since interactions between poly(urea-formaldehyde) networks and water were anticipated due to the presence of amide and hydroxyl functional groups on the shells, which can form hydrogen bonding with water molecules [
39]. The swelling increased significantly in the first 24 h and reached a plateau after that for 100T-U and 100T-ME, indicating that PUF microcapsules reached the equilibrium swollen size, a thermodynamically favorable condition, considerably fast. It is important to highlight the microcapsules swell in response to the differences in osmotic pressure across their shells, which becomes negligible as the capsule reaches the equilibrium swollen size [
40]. It is also important to observe that the swelling process is a dynamic phenomenon characterized by the inward diffusion of water and the outward diffusion of cargo through the solvent-permeable shell. This is evidenced by the increase in the microcapsule diameter and the optical micrographs showing microcapsules with their core only partially filled with the cargo (marked in orange). The 100T-MA group reached the plateau a little later, with 1-week incubation, which is probably related to increased porosity in their shells due to heterogeneities in the polymeric network, as discussed earlier. In addition, all the groups experienced an increase in the microcapsule diameter but a decrease in cargo weight. This is because the density of water is approximately 10% lower than TEGDMA density (~1.092 g/mL at 25 °C). Altogether, these findings indicate that the osmotic pressure inside the capsule was significantly higher than the outer solution (hypotonic conditions), and the equilibrium was reached at the expense of water influx and cargo efflux through the solvent-permeable microcapsule shell [
40,
41]. The 100T-MA and 100T-U groups showed a significant rupture of microcapsules after 4 weeks of incubation, as evidenced by the presence of free orange drops of cargo and polymeric sheets dispersed into the background of the optical micrographs (
Figure 6 and
Figure 7). This was expected, as the 4-week incubation reflects the cumulative effects of the water plasticizing effect during the entire incubation period. This also indicates that the larger swollen capsules did not withstand the deformation and burst after 3 weeks [
41]. The burst of these microcapsules was caused when their internal pressure exceeded the failure strength of the shell material [
41]. It is important to note that for both the 100T-MA and 100T-U groups, the diameters of their microcapsules after 4 weeks are like those of the control. Although some microcapsules are capable of deswelling, this is unlikely to be the case in this study. This similarity arises since the larger capsules, more susceptible to breaking, burst after 3 weeks, and the intact remaining capsules that could have their diameter assessed were the smaller ones. In general, 100T-ME microcapsules demonstrated higher stability and mechanical properties than 100T-MA and 100T-U, as shown through their physical integrity and the preservation of cargo highlighted in the optical micrographs and stable cargo weight variation profile. This underscores the superior mechanical properties of their polymeric shells and showcases their resilience against solvent effects [
40].
In PBS, the swelling of the microcapsules was as noticeable as that seen in water incubation. However, differently from water-incubated groups, it was mainly related to the swelling of the shells rather than outer solution influx and cargo efflux. It is evidenced by the preservation of the shell core that, despite the capsule swelling, was maintained well delimited, regular, and spherical. Surrounding the undisturbed core there is a distinct asymmetrical clear halo, which is characteristic of shell swelling and typically seen in microcapsules with uneven shell thickness [
40,
41]. To confirm that the microcapsule expansion relied solely on the swelling of the shell, 10 mg of 100T-ME microcapsules was dispersed in 1 mL of 0.1 M PBS, and a single microcapsule with a diameter of 163 µm was observed to monitor its response in real time via optical microscopy (
Figure 9A). After 2 min of incubation, a halo began forming on the bottom of the microcapsule and expanded quickly, reaching its maximum dimension after 15 min. During this time span, the microcapsule volume increased by about 12%, reaching a diameter of 185.5 µm. After 15 min, as the PBS evaporated, the halo experienced a decreasing change in volume, fading away after 50 min. Throughout the monitoring period, the cargo remained undisturbed, and no efflux was observed during the microcapsule shell expansion and regression processes. This experiment demonstrated that shell polymer is highly tough and capable of enduring a large level of mechanical strain [
41]. It also suggested an unexpected degree of ionization occurring at the PUF network [
40]. When dispersed in a polar solvent enriched with salt, such as PBS, an unbalanced concentration of ions is observed among the microcapsule core, the shell, and the outer solution. Under hypertonic conditions, where the osmotic pressure of the microcapsule inner core is lower than the salt-enriched external solution, the osmotic pressure difference across the shell should lead cargo to diffuse out of the microcapsules [
41]. However, the experiments showed the opposite behavior. A possible explanation for this may be based on the ionic behavior of the PUF shells, which might become charged by dissociating counterions from the polymer backbones and swell by absorbing the solvent into the polymer network [
42]. This redistribution of the mobile counterions of the shells and the co-ions of the outer solution affects the swelling behavior of the microcapsules until a local Donnan equilibrium is established among the core, shell, and external media [
40]. While the PUF network and the PBS are individually neutral, their mixture results in a 0.5-unit drop in pH (
Figure 9B). This observation suggests the potential transfer of ions between the microcapsule and the external media. It is important to highlight that the microcapsule swells to an equilibrium size because it is not capable of sustaining a non-neutral osmotic pressure. As the equilibrium swollen size is reached, thermodynamic equilibrium is achieved [
40]. The leakage of the cargo, noticed in the optical micrographs, is the eventual result of the shell ruptured at its thinnest part, i.e., its weak spot [
40]. The microcapsules most adversely affected by the incubation in PBS were the ones synthesized via the ultrasonication technique (
Figure 7). This was expected given the significantly smaller size of the microcapsules, which means a higher surface area-to-volume ratio in comparison to the other two groups and, ultimately, more susceptibility to interact with the external solution.
The results for 80T/20D groups incubated at different conditions are depicted in
Figure 10,
Figure 11 and
Figure 12. In general, the morphological changes as a function of the storage conditions followed the same trends observed in 100T groups. In other words, wrinkles and shrinkage are observed as the microcapsules are stored in dry conditions at 4 °C; the dynamic influx of water and the efflux of cargo are observed at water incubation; and shell swelling is observed at PBS incubation. However, the 80T/20D microcapsules demonstrated superior stability and resistance to incubation, irrespective of integrity preservation, diameter, and cargo weight variation. This is particularly evident when comparing the magnetically stirred groups (
Figure 6 and
Figure 10). The anticipated increased stability in water or PBS can be attributed to the highly hydrophilic nature of DMAM. Beyond the advantages discussed earlier regarding microemulsification stability and encapsulation efficiency, DMAM may have diminished the permeability of the microcapsule shell to external media due to its water affinity. This may have posed a challenge for water penetration, ultimately minimizing cargo efflux and shell plasticization, hydrolysis, and swelling [
43]. Furthermore, more hydrophilic cargo may act as stabilizing agents by preventing the coalescence and aggregation of microcapsules, thereby enhancing their stability in aqueous media. In fact, DMAM-containing microcapsules exhibited a more dispersed and free-flowing aspect compared to 100T microcapsules, which tended to form agglomerates. This phenomenon is linked to the water-attracting nature of the cargo, creating a barrier between adjacent microcapsules and increasing their dispersion. Lastly, acrylamides have been added into poly(urea-formaldehyde) polymeric networks as a strategy to increase mechanical strength and water resistance [
44,
45,
46]. The DMAM that migrated to the water phase and dissolved with the shell precursors may have been incorporated into the shell networks, thereby enhancing their mechanical performance. This optimization might likely contribute to increased stability and resistance to incubation for these microcapsules. A comprehensive investigation of this potential phenomenon will be conducted in a separate study. In contrast to the magnetic and mechanically stirred groups, the 80T/20D-U microcapsules exhibited a significant number of empty and broken microcapsules and droplets of cargo, as evidenced by the optical micrographs (
Figure 11A). The variation in size and cargo weight was also pronounced, as shown in
Figure 11B. This group was the most affected by the Ostwald Ripening effect, which is related to shells with extensive variations in thickness, polymeric structural irregularities, increased porosity, and points of stress concentration [
30]. It may have compromised the mechanical strength of the PUF polymeric networks, jeopardizing their stability and ability to withstand the osmotic challenge imposed by aqueous incubation.