Unveiling the Cultivation of Nostoc sp. under Controlled Laboratory Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
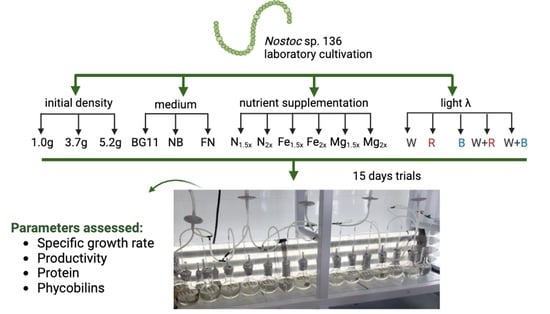
2.1. Culture Conditions
- C1—biomass concentration at time t1, C2—biomass concentration at time t2, Δt—difference in time.
2.2. Biochemical Analysis
- [PC]—C-Phycocyanin
- [APC]—Allophycocyanin
- [PE]—C-Phycoerythrin
- OD(615 nm)—Absorbance of the sample at wavelength of 615 nm
- OD(652 nm)—Absorbance of the sample at wavelength of 652 nm
- OD(562 nm)—Absorbance of the sample at wavelength of 562 nm
2.3. Statistical Analysis
3. Results
3.1. Culture Conditions
3.2. Biochemical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berman-Frank, I.; Lundgren, P.; Falkowski, P. Nitrogen Fixation and Photosynthetic Oxygen Evolution in Cyanobacteria. Res. Microbiol. 2003, 154, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Rucker, H.R.; Kaçar, B. Enigmatic Evolution of Microbial Nitrogen Fixation: Insights from Earth’s Past. Trends Microbiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-Based Products for the Food and Feed Sector: An Outlook for Europe; Institute for Prospective Technological Studies Fashion Nutraceuticals, Publications Office of the European Union: Luxembourg, 2014; ISBN 9789279340376. [Google Scholar]
- Flores, C.; Tamagnini, P. Looking Outwards: Isolation of Cyanobacterial Released Carbohydrate Polymers and Proteins. J. Vis. Exp. 2019, 147, e59590. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Olivieri, G.; de Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.M.; Kleinegris, D.M.M.; Wijffels, R.H.; Barbosa, M.J. Towards Industrial Products from Microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef]
- Santini, G.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Plant Biostimulants from Cyanobacteria: An Emerging Strategy to Improve Yields and Sustainability in Agriculture. Plants 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Thanuja, G.; Ramasamy, A.; Karthikeyan, S. Microalgae and cyanobacteria: Role and applications in agriculture. In Applied Algal Biotechnology; Arumugam, M., Kathiresan, S., Subramani, N., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; ISBN 9781536175240. [Google Scholar]
- Laroche, C. Exopolysaccharides from Microalgae and Cyanobacteria: Diversity of Strains, Production Strategies, and Applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef]
- Liang, F.; Englund, E.; Lindberg, P.; Lindblad, P. Engineered Cyanobacteria with Enhanced Growth Show Increased Ethanol Production and Higher Biofuel to Biomass Ratio. Metab. Eng. 2018, 46, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Singh, T.D.M.B.; Kumar, A. Cyanobacteria: Applications in Biotechnology. In Cyanobacteria from Basic Science to Applications; Mishra, A.K., Tiwari, D.N., Rai, A.N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 327–346. [Google Scholar]
- Kondi, V.; Sabbani, V.; Alluri, R.; Karumuri, T.S.K.; Chawla, P.; Dasarapu, S.; Tiwari, O.N. Cyanobacteria as Potential Bio Resources for Multifaceted Sustainable Utilization. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 73–87. [Google Scholar]
- Christman, H.D.; Campbell, E.L.; Meeks, J.C. Global Transcription Profiles of the Nitrogen Stress Response Resulting in Heterocyst or Hormogonium Development in Nostoc punctiforme. J. Bacteriol. 2011, 193, 6874–6886. [Google Scholar] [CrossRef]
- Zhao, J.; Peter Wolk, C. Developmental Biology of Heterocysts. In Myxobacteria: Multicellularity and Differentiation; Whitworth, D.E., Ed.; ASM Press: Washington, DC, USA, 2007; pp. 397–418. [Google Scholar]
- Ahad, R.I.A.; Goswami, S.; Syiem, M.B. Biosorption and Equilibrium Isotherms Study of Cadmium Removal by Nostoc muscorum Meg 1: Morphological, Physiological and Biochemical Alterations. 3 Biotech 2017, 7, 104. [Google Scholar] [CrossRef]
- Whitton, B.A.; Potts, M. (Eds.) The Ecology of Cyanobacteria; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; ISBN 0-7923-4735-8. [Google Scholar]
- Trentin, G.; Piazza, F.; Carletti, M.; Zorin, B.; Khozin-Goldberg, I.; Bertucco, A.; Sforza, E. Fixing N2 into Cyanophycin: Continuous Cultivation of Nostoc sp. PCC 7120. Appl. Microbiol. Biotechnol. 2023, 107, 97–110. [Google Scholar] [CrossRef]
- Gheda, S.F.; Ahmed, D.A. Improved Soil Characteristics and Wheat Germination as Influenced by Inoculation of Nostoc kihlmani and Anabaena cylindrica. Rend. Lincei 2015, 26, 121–131. [Google Scholar] [CrossRef]
- Kollmen, J.; Strieth, D. The Beneficial Effects of Cyanobacterial Co-Culture on Plant Growth. Life 2022, 12, 223. [Google Scholar] [CrossRef]
- Cottas, A.G.; Teixeira, T.A.; Cunha, W.R.; Ribeiro, E.J.; de Souza Ferreira, J. Effect of Glucose and Sodium Nitrate on the Cultivation of Nostoc sp. PCC 7423 and Production of Phycobiliproteins. Braz. J. Chem. Eng. 2022, 39, 1–9. [Google Scholar] [CrossRef]
- Touloupakis, E.; Zittelli, G.C.; Benavides, A.M.S.; Torzillo, G. Growth and Photosynthetic Performance of Nostoc linckia (Formerly N. calcicola) Cells Grown in BG11 and BG110 Media. Photochem. Photobiol. Sci. 2022, 22, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jia, S.; Dai, Y. Growth Characteristics of the Cyanobacterium Nostoc flagelliforme in Photoautotrophic, Mixotrophic and Heterotrophic Cultivation. J. Appl. Phycol. 2009, 21, 127–133. [Google Scholar] [CrossRef]
- Ma, R.; Lu, F.; Bi, Y.; Hu, Z. Effects of Light Intensity and Quality on Phycobiliprotein Accumulation in the Cyanobacterium Nostoc sphaeroides Kützing. Biotechnol. Lett. 2015, 37, 1663–1669. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Rearte, T.A.; Neori, A.; Masojídek, J.; Bonomi-Barufi, J.; Álvarez-Gómez, F.; Ranglová, K.; Carmo da Silva, J.; Abdala, R.; Gómez, C.; et al. A New Approach for Cultivating the Cyanobacterium Nostoc calcicola (MACC-612) to Produce Biomass and Bioactive Compounds Using a Thin-Layer Raceway Pond. Algal Res. 2021, 59, 102421. [Google Scholar] [CrossRef]
- Walther, J.; Erdmann, N.; Stoffel, M.; Wastian, K.; Schwarz, A.; Strieth, D.; Muffler, K.; Ulber, R. Passively Immobilized Cyanobacteria Nostoc species BB 92.2 in a Moving Bed Photobioreactor (MBPBR): Design, Cultivation, and Characterization. Biotechnol. Bioeng. 2022, 119, 1467–1482. [Google Scholar] [CrossRef]
- Strieth, D.; Weber, A.; Robert, J.; Stiefelmaier, J.; Kollmen, J.; Volkmar, M.; Lakatos, M.; Jordan, V.; Muffler, K.; Ulber, R. Characterization of an Aerosol-Based Photobioreactor for Cultivation of Phototrophic Biofilms. Life 2021, 11, 1046. [Google Scholar] [CrossRef]
- Fischer, D.; Schlösser, U.G.; Pohl, P. Exopolysaccharide Production by Cyanobacteria Grown in Closed Photobioreactors and Immobilized Using White Cotton Towelling. J. Appl. Phycol. 1997, 9, 205–213. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Khangembam, R.; Shamjetshabam, M.; Sharma, A.S.; Oinam, G.; Brand, J.J. Characterization and Optimization of Bioflocculant Exopolysaccharide Production by Cyanobacteria Nostoc sp. BTA97 and Anabaena sp. BTA990 in Culture Conditions. Appl. Biochem. Biotechnol. 2015, 176, 1950–1963. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, H.; Zhu, Z.; Gao, X. The Effects of the Exopolysaccharide and Growth Rate on the Morphogenesis of the Terrestrial Filamentous Cyanobacterium Nostoc flagelliforme. Biol. Open 2017, 6, 1329–1335. [Google Scholar] [CrossRef]
- Reis, A.; Mendes, A.; Lobo-Fernandes, H.; Empis, J.A.; Novais, J.M. Production, Extraction and Purification of Phycobiliproteins from Nostoc sp. Bioresour. Technol. 1998, 66, 181–187. [Google Scholar] [CrossRef]
- Lee, N.K.; Oh, H.M.; Kim, H.S.; Ahn, C.Y. Higher Production of C-Phycocyanin by Nitrogen-Free (Diazotrophic) Cultivation of Nostoc sp. NK and Simplified Extraction by Dark-Cold Shock. Bioresour. Technol. 2017, 227, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.G.; Jia, S.R.; Wu, Y.K.; Yan, R.R.; Lin, Y.H.; Zhao, D.X.; Han, P.P. Effect of culture conditions on the physicochemical properties and antioxidant activities of polysaccharides from Nostoc flagelliforme. Carbohydr. Polym. 2018, 198, 426–433. [Google Scholar] [CrossRef]
- Bhati, R.; Mallick, N. Production and Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Co-Polymer by a N2-Fixing Cyanobacterium, Nostoc muscorum Agardh. J. Chem. Technol. Biotechnol. 2012, 87, 505–512. [Google Scholar] [CrossRef]
- Sharma, L.; Mallick, N. Accumulation of Poly-β-Hydroxybutyrate in Nostoc muscorum: Regulation by PH, Light–Dark Cycles, N and P Status and Carbon Sources. Bioresour. Technol. 2005, 96, 1304–1310. [Google Scholar] [CrossRef]
- Roshan, S.K.; Farhangi, M.; Emtyazjoo, M.; Rabbani, M. Effects of Solar Radiation on Pigmentation and Induction of a Mycosporine-like Amino Acid in Two Cyanobacteria, Anabaena sp. and Nostoc sp. ISC26. Eur. J. Phycol. 2015, 26, 173–181. [Google Scholar] [CrossRef]
- Feng, Y.-N.; Zhang, Z.-C.; Feng, J.-L.; Qiu, B.-S. Effects of UV-B Radiation and Periodic Desiccation on the Morphogenesis of the Edible Terrestrial Cyanobacterium Nostoc flagelliforme. Appl. Environ. Microbiol. 2012, 78, 7075–7081. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; El-Shouny, W.A.; Osman, M.E.H.; El-Gammal, E.W.E. Growth and Heavy Metals Removal Efficiency of Nostoc muscorum and Anabaena subcylindrica in Sewage and Industrial Wastewater Effluents. Environ. Toxicol. Pharmacol. 2005, 19, 357–365. [Google Scholar] [CrossRef]
- El Shafay, S.M.; Gaber, A.; Alsanie, W.F.; Elshobary, M.E. Influence of Nutrient Manipulation on Growth and Biochemical Constituent in Anabaena variabilis and Nostoc muscorum to Enhance Biodiesel Production. Sustainability 2021, 13, 9081. [Google Scholar] [CrossRef]
- McFadden, G.I.; Melkonian, M. Use of Hepes Buffer for Microalgal Culture Media and Fixation for Electron Microscopy. Phycologia 1986, 25, 551–557. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Mouga, T.; Simões, F.; Moreira, V.; Martins, A.; Ferreira, C.; Ramos, R.; Afonso, C. Producing Cyanobacteria to Use as Biostimulants. In Proceedings of the 2nd International Conference on Water Energy Food and Sustainability (ICoWEFS 2022), Portalegre, Portugal, 10–12 May 2022; Springer: Cham, Switzerland, 2023; Volume 34, pp. 26–35, ISBN 9783030893088. [Google Scholar]
- Levasseur, M.; Thompson, P.A.; Harrison, P.J. Physiological Acclimation of Marine Phytoplankton to Different Nitrogen Sources. J. Phycol. 1993, 29, 587–595. [Google Scholar] [CrossRef]
- Bastos, C.R.V.; Maia, I.B.; Pereira, H.; Navalho, J.; Varela, J.C.S. Optimisation of Biomass Production and Nutritional Value of Two Marine Diatoms (Bacillariophyceae), Skeletonema costatum and Chaetoceros calcitrans. Biology 2022, 11, 594. [Google Scholar] [CrossRef]
- Olofsson, M.; Lamela, T.; Nilsson, E.; Bergé, J.P.; del Pino, V.; Uronen, P.; Legrand, C. Seasonal Variation of Lipids and Fatty Acids of the Microalgae Nannochloropsis oculata Grown in Outdoor Large-Scale Photobioreactors. Energies 2012, 5, 1577–1592. [Google Scholar] [CrossRef]
- Concórdio-Reis, P.; Cardeira, M.; Macedo, A.C.; Ferreira, S.S.; Serra, A.T.; Coimbra, M.A.; Amorim, A.; Reis, M.A.M.; Freitas, F. Novel Exopolysaccharide Produced by the Marine Dinoflagellate Heterocapsa AC210: Production, Characterization, and Biological Properties. Algal Res. 2023, 70, 103014. [Google Scholar] [CrossRef]
- Carneiro, J.; Gomes, S.; Freitas, M.; Afonso, C.; Mouga, T. Growth of Arthrospira platensis under laboratory and outdoor conditions: Assessment of the effects of light and different nutrient media. Front. Mar. Sci. 2018, 5, 2022. [Google Scholar] [CrossRef]
- Wishkerman, A.; Wishkerman, E. Application Note: A Novel Low-Cost Open-Source LED System for Microalgae Cultivation. Comput. Electron. Agric. 2017, 132, 56–62. [Google Scholar] [CrossRef]
- Davidson, M.W. Fundamentals of Light-Emitting Diodes. Zeiss Microscopy 2008, 1–12. Available online: https://zeiss-campus.magnet.fsu.edu/print/lightsources/leds-print.html (accessed on 24 April 2024).
- Parimi, N.S.; Singh, M.; Kastner, J.R.; Das, K.C.; Forsberg, L.S.; Azadi, P. Optimization of Protein Extraction from Spirulina platensis to Generate a Potential Co-Product and a Biofuel Feedstock with Reduced Nitrogen Content. Front. Energy Res. 2015, 3, 30. [Google Scholar] [CrossRef]
- Martin, I.; Cabán-Hernández, K.; Figueroa-Santiago, O.; Espino, A.M. Fasciola hepatica Fatty Acid Binding Protein Inhibits TLR4 Activation and Suppresses the Inflammatory Cytokines Induced by Lipopolysaccharide In Vitro and In Vivo. J. Immunol. 2015, 194, 3924–3936. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Bogorad, L. Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Sholkamy, E.N.; El-Komy, H.; Al-Arfaj, A.A.; Abdel-Megeed, A.; Mostafa, A.A. Potential Role of Nostoc muscorum and Nostoc rivulare as Biofertilizers for the Enhancement of Maize Growth under Different Doses of N-Fertilizer. Afr. J. Microbiol. Res. 2012, 6, 7435–7448. [Google Scholar] [CrossRef]
- Maqubela, M.P.; Mnkeni, P.N.S.; Issa, O.M.; Pardo, M.T.; D’Acqui, L.P. Nostoc Cyanobacterial Inoculation in South African Agricultural Soils Enhances Soil Structure, Fertility, and Maize Growth. Plant Soil 2009, 315, 79–92. [Google Scholar] [CrossRef]
- Chai, Y.; Cai, P.; Gillor, O.; Kuramae, E.E.; A Costa, O.Y.; Raaijmakers, J.M. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 337094. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Cultivation of Microalgae and Cyanobacteria: Effect of Operating Conditions on Growth and Biomass Composition. Molecules 2020, 25, 2834. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.-Y.; Al-Hammady, M.A.M.; et al. Cyanobacteria—From the Oceans to the Potential Biotechnological and Biomedical Applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Noworyta, A. Evaluation of Spirulina sp. Growth in Photoautotrophic, Heterotrophic and Mixotrophic Cultures. Enzym. Microb. Technol. 2004, 34, 461–465. [Google Scholar] [CrossRef]
- Baracho, D.H.; Lombardi, A.T. Study of the Growth and Biochemical Composition of 20 Species of Cyanobacteria Cultured in Cylindrical Photobioreactors. Microb. Cell Fact. 2023, 22, 36. [Google Scholar] [CrossRef]
- Straka, L.; Rittmann, B.E. Effect of Culture Density on Biomass Production and Light Utilization Efficiency of Synechocystis sp. PCC 6803. Biotechnol. Bioeng. 2018, 115, 507–511. [Google Scholar] [CrossRef]
- Van Khanh, N.; Thi Diem, N.; Tuyet Nhan, L.T.; Cu, P.V.; Khanh Van, T.Q.; Thi Hoan, N.T. The Effects of Nutritional Media and Initial Cell Density on the Growth and Development of Spirulina platensis. J. Agric. Sci. Technol. A 2017, 7, 60–67. [Google Scholar] [CrossRef]
- Hewes, C.D. Not All Culture Is Created Equal: A Comparative Study in Search of the Most Productive Cultivation Methodology. Algal Res. 2015, 12, 561–568. [Google Scholar] [CrossRef]
- Aranda-Vega, Y.; Bhatt, P.; Huang, J.-Y.; Brown, P.; Bhasin, A.; Hussain, A.S.; Simsek, H. Biodegradability and Bioavailability of Dissolved Substances in Aquaculture Effluent: Performance of Indigenous Bacteria, Cyanobacteria, and Green Microalgae. Environ. Pollut. 2024, 345, 123468. [Google Scholar] [CrossRef] [PubMed]
- Cavet, J.S.; Borrelly, G.P.M.; Robinson, N.J. Zn, Cu and Co in Cyanobacteria: Selective Control of Metal Availability. FEMS Microbiol. Rev. 2003, 27, 165–181. [Google Scholar] [CrossRef]
- Dawar, S.; Mohanty, P.; Behera, B.K. Sustainable Hydrogen Production in the Cyanobacterium Nostoc sp. ARM 411 Grown in Fructose- and Magnesium Sulphate-Enriched Culture. World J. Microbiol. Biotechnol. 1999, 15, 329–332. [Google Scholar] [CrossRef]
- Che, R.Q.; Wang, Q.M.; Huang, L.; Zhao, P.; Yu, X.Y. Effects of Additional Mg2+ on the Growth and Lipid Accumulation of Monoraphidium sp. FXY-10 under Mixotrophic Conditions. Adv. Mater. Res. 2014, 860–863, 920–927. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Prášil, O.; El-Mohsnawy, E. Physiological and Spectroscopical Changes of the Thermophilic Cyanobacterium Synechococcus elongatus under Iron Stress and Recovery Culture. Acta Physiol. Plant. 2021, 43, 72. [Google Scholar] [CrossRef]
- Halac, S.R.; Ruibal-Conti, A.L.; Mengo, L.d.V.; Ullmer, F.; Cativa, A.; Bazan, R.; Rodriguez, M.I. Effect of Iron Availability on the Growth and Microcystin Content of Natural Populations of Microcystis spp. from Reservoirs in Central Argentina: A Microcosm Experiment Approach. Phycology 2023, 3, 168–185. [Google Scholar] [CrossRef]
- Rueter, J.G.; Ohki, K.; Fujita, Y. The Effect of Iron Nutrition on Photosynthesis and Nitrogen Fixation in Cultures of Trichodesmium (Cyanophyceae). J. Phycol. 1990, 26, 30–35. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative Stress in Cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef]
- Mohanty, B.; Majedi, S.M.; Pavagadhi, S.; Te, S.H.; Boo, C.Y.; Gin, K.Y.H.; Swarup, S. Effects of Light and Temperature on the Metabolic Profiling of Two Habitat-Dependent Bloom-Forming Cyanobacteria. Metabolites 2022, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.G.; Waldeck, P.; Sykora, S.; Braune, S.; Petrick, I.; Küpper, J.H.; Jung, F. Influence of Different Light-Emitting Diode Colors on Growth and Phycobiliprotein Generation of Arthrospira platensis. Life 2022, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Bonomi-Barufi, J.; Vega, J.; Abdala-Díaz, R.; Vasconcelos, V.; Guedes, A.C.; Figueroa, F.L. Light Quality Triggers Biochemical Modulation of Cyanobium sp.—Photobiology as Tool for Biotechnological Optimization. J. Appl. Phycol. 2020, 32, 2851–2861. [Google Scholar] [CrossRef]
- Kim, N.N.; Shin, H.S.; Park, H.G.; Lee, J.; Kil, G.S.; Choi, C.Y. Profiles of Photosynthetic Pigment Accumulation and Expression of Photosynthesis-Related Genes in the Marine Cyanobacteria Synechococcus sp.: Effects of LED Wavelengths. Biotechnol. Bioprocess Eng. 2014, 19, 250–256. [Google Scholar] [CrossRef]
- Slocombe, S.P.; Ross, M.; Thomas, N.; McNeill, S.; Stanley, M.S. A Rapid and General Method for Measurement of Protein in Micro-Algal Biomass. Bioresour. Technol. 2013, 129, 51–57. [Google Scholar] [CrossRef]







| Trial 1. Inoculum Concentration | ||||
| Inoculum concentration | mBG11 (1.0 g.L−1) | mBG11 (3.7 g.L−1) | mBG11 (5.2 g.L−1) | |
| Trial 2. Medium selection | ||||
| Medium | mBG11 (10 mL.L−1) | Nutribloom (1.25 mL.L−1) | FloraNova (0.25 mL.L−1) | |
| Trial 3. Nutrients supplementation | ||||
| Nitrogen | 1× (1.5000 g.L−1) | 1.5× (2.2500 g.L−1) | 2× (3.0000 g.L−1) | |
| Iron | 1× (0.0030 g.L−1) | 1.5× (0.0045 g.L−1) | 2× (0.0060 g.L−1) | |
| Magnesium | 1× (0.0750 g.L−1) | 1.5× (0.1125 g.L−1) | 2× (0.1500 g.L−1) | |
| Trial 4. Light conditions | ||||
| Single LED | Blue—peak at 465 nm | White (450 and 550–620 nm) | Red—peak at 635 nm | |
| Combined LED | White and Blue (440–470 + 550–620 nm) | White and red (550–640 nm) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouga, T.; Pereira, J.; Moreira, V.; Afonso, C. Unveiling the Cultivation of Nostoc sp. under Controlled Laboratory Conditions. Biology 2024, 13, 306. https://doi.org/10.3390/biology13050306
Mouga T, Pereira J, Moreira V, Afonso C. Unveiling the Cultivation of Nostoc sp. under Controlled Laboratory Conditions. Biology. 2024; 13(5):306. https://doi.org/10.3390/biology13050306
Chicago/Turabian StyleMouga, Teresa, Jéssica Pereira, Vitória Moreira, and Clélia Afonso. 2024. "Unveiling the Cultivation of Nostoc sp. under Controlled Laboratory Conditions" Biology 13, no. 5: 306. https://doi.org/10.3390/biology13050306