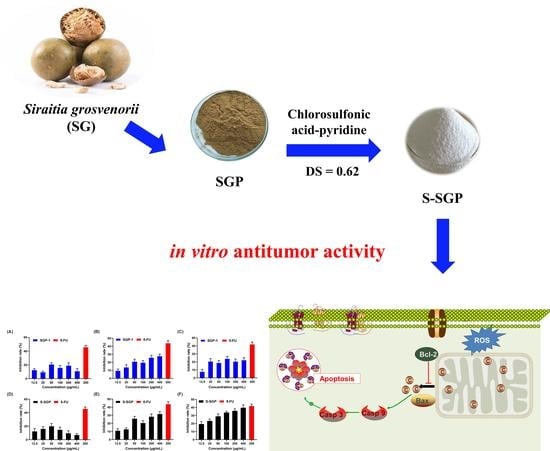
Structure Characterization, In Vitro Antioxidant and Anti-Tumor Activity of Sulfated Polysaccharide from Siraitia grosvenorii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Isolation and Purification of the Siraitia Grosvenorii Polysaccharide
2.3. Characterization of Siraitia Grosvenorii Polysaccharide (SGP-1)
2.3.1. Analysis of the Polysaccharide Content
2.3.2. Determination of Molecular Weight (Mw) and Monosaccharide Compositions
2.3.3. Ultraviolet (UV) and Fourier Transform Infrared (FT-IR) Spectra Analysis
2.3.4. Morphology Analysis
2.4. Sulfated Modification of SGP-1
2.5. Characterization of Sulfated Polysaccharide (S-SGP)
2.5.1. Degree of Substitution (DS)
2.5.2. Analysis of the Polysaccharide Content, Mw, FT-IR and Morphology Analysis
2.6. In Vitro Antioxidant Assay
2.6.1. OH Scavenging Activity
2.6.2. DPPH Scavenging Activity
2.6.3. O2−∙ Scavenging Activity
2.7. Anti-Cancer and Apoptosis Assay
2.7.1. Cell Lines and Cultures
2.7.2. Growth Inhibition Assay
2.7.3. Apoptosis of A549 Cells
2.7.4. Apoptosis Mechanism of A549 Cells
3. Results and Discussion
3.1. Characterization of SGP-1
3.2. Characterization of S-SGP
3.3. In Vitro Antioxidant Activity
3.4. Growth Inhibition of HepG2 Cells, MDA-MB-231 Cells and A549 Cells by S-SGP
3.5. S-SGP Induces Apoptosis in A549 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Chen, X.; Gong, P.; Wang, M.; Yao, W.; Yang, W.; Chen, F. In vitro digestion and fecal fermentation of Siraitia grosvenorii polysaccharide and its impact on human gut microbiota. Food Funct. 2022, 13, 9443–9458. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P.; Wang, M.; Yao, W.; Yang, W.; Chen, F. Effects of simulated saliva-gastrointestinal digestion on the physicochemical properties and bioactivities of Siraitia grosvenorii polysaccharides. Int. J. Food Sci. Technol. 2022, 57, 4495–4506. [Google Scholar] [CrossRef]
- Gong, P.; Cui, D.; Guo, Y.; Wang, M.; Wang, Z.; Huang, Z.; Yang, W.; Chen, F.; Chen, X. A novel polysaccharide obtained from Siraitia grosvenorii alleviates inflammatory responses in a diabetic nephropathy mouse model via the TLR4-NF-κB pathway. Food Funct. 2021, 12, 9054–9065. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P.; Li, Z.; Wu, Y.; Zhang, J.; Wang, J.; Yao, W.; Yang, W.; Chen, F. Advances in the mechanisms of polysaccharides in alleviating depression and its complications. Phytomedicine 2023, 109, 154566–154574. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-P.; Jiang, T.; Hu, X.-B.; Qiao, X.-H.; Tuo, Q.-H. Effect of Siraitia Grosvenorii Polysaccharide on Glucose and Lipid of Diabetic Rabbits Induced by Feeding High Fat/High Sucrose Chow. Exp. Diabetes Res. 2007, 2007, 067435. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-M.; Pan, L.-C.; Zhang, L.-J.; Yin, Y.; Zhu, Z.-Y.; Sun, H.-Q.; Liu, C.-Y. Chemical structure and antioxidant activity of a polysaccharide from Siraitia grosvenorii. Int. J. Biol. Macromol. 2020, 165, 1900–1910. [Google Scholar] [CrossRef]
- Gong, P.; Guo, Y.; Chen, X.; Cui, D.; Wang, M.; Yang, W.; Chen, F. Structural Characteristics, Antioxidant and Hypoglycemic Activities of Polysaccharide from Siraitia grosvenorii. Molecules 2022, 27, 4192. [Google Scholar] [CrossRef]
- Meng, X.; Edgar, K.J. “Click” reactions in polysaccharide modification. Prog. Polym. Sci. 2016, 53, 52–85. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T.; Koschella, A. Esterification of Polysaccharides; Springer Science & Business Media: Berlin, Germany, 2006. [Google Scholar]
- Lee, W.-K.; Ho, C.-L. Ecological and evolutionary diversification of sulphated polysaccharides in diverse photosynthetic lineages: A review. Carbohydr. Polym. 2022, 277, 118764. [Google Scholar] [CrossRef]
- Bedini, E.; Laezza, A.; Parrilli, M.; Iadonisi, A. A review of chemical methods for the selective sulfation and desulfation of polysaccharides. Carbohydr. Polym. 2017, 174, 1224–1239. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated modification of polysaccharides: Synthesis, characterization and bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Li, J.; Chi, Z.; Yu, L.; Jiang, F.; Liu, C. Sulfated modification, characterization, and antioxidant and moisture absorption/retention activities of a soluble neutral polysaccharide from Enteromorpha prolifera. Int. J. Biol. Macromol. 2017, 105, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Vasilieva, N.Y.; Malyar, Y.N.; Karacharov, A.A.; Kondrasenko, A.A.; Levdanskiy, A.V.; Borovkova, V.S.; Miroshnikova, A.V.; Issaoui, N.; Kazachenko, A.S.; et al. Sulfation of arabinogalactan with ammonium sulfamate. Biomass Convers. Biorefinery 2022, 102, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Xu, X.; Lin, Y.; Cheung, P.C.K.; Kennedy, J.F. Comparison on chain stiffness of a water-insoluble (1→3)-α-d-glucan isolated from Poria cocos mycelia and its sulfated derivative. Carbohydr. Polym. 2005, 59, 257–263. [Google Scholar] [CrossRef]
- Zong, A.; Liu, Y.; Zhang, Y.; Song, X.; Shi, Y.; Cao, H.; Liu, C.; Cheng, Y.; Jiang, W.; Du, F.; et al. Anti-tumor activity and the mechanism of SIP-S: A sulfated polysaccharide with anti-metastatic effect. Carbohydr. Polym. 2015, 129, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, A.; Govindan, S.; Ramani, P.; Subbaiah, K.A.; Sathianarayanan, S.; Venkidasamy, B.; Thiruvengadam, M.; Rebezov, M.; Shariati, M.A.; Lorenzo, J.M.; et al. Antioxidant, Anti-Tumour, and Anticoagulant Activities of Polysaccharide from Calocybe indica (APK2). Antioxidants 2022, 11, 1694. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Bi, D.; Huang, J.; Cao, J.; Yao, L.; Guo, W.; Zhang, Z.; Wu, Y.; Xu, H.; Hu, Z.; Xu, X. Preparation, characterization and immunomodulatory effects of unsaturated sulfated oligoguluronic acid. Carbohydr. Polym. 2023, 301, 120370. [Google Scholar] [CrossRef]
- You, Y.; Song, H.; Wang, L.; Peng, H.; Sun, Y.; Ai, C.; Wen, C.; Zhu, B.; Song, S. Structural characterization and SARS-CoV-2 inhibitory activity of a sulfated polysaccharide from Caulerpa lentillifera. Carbohydr. Polym. 2022, 280, 119006. [Google Scholar] [CrossRef]
- Xian, H.; Wang, P.; Jing, H.; Chen, G.-Q.; Cheng, D.-F.; Ji, F.; Song, S.; Zhang, L. Comparative study of components and anti-oxidative effects between sulfated polysaccharide and its iron complex. Int. J. Biol. Macromol. 2018, 118, 1303–1309. [Google Scholar] [CrossRef]
- Hao, H.; Cui, C.; Xing, Y.; Jia, X.; Ma, B.; Kang, W.; Li, T.; Gao, M.; Xu, C. Sulfation of the extracellular polysaccharide from the edible fungus Stropharia rugosoannulata with its antioxidant activity. J. Futur. Foods 2023, 3, 37–42. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Jia, S.; Huang, L.; Wang, Z.; Li, C.; Xie, M. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264. 7. Int. J. Biol. Macromol. 2017, 98, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Chen, F.; Cui, D.; Wang, M. Advances in the in vitro digestion and fermentation of polysaccharides. Int. J. Food Sci. Technol. 2021, 56, 4970–4982. [Google Scholar] [CrossRef]
- Gong, B.; Cheng, L.; Gilbert, R.G.; Li, C. Distribution of short to medium amylose chains are major controllers of in vitro digestion of retrograded rice starch. Food Hydrocoll. 2019, 96, 634–643. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Dong, Y.; Fang, Y.; Gao, W.; Kang, X.; Liu, P.; Yan, S.; Cui, B.; El-Aty, A.A. Effects of different moisture contents on the structure and properties of corn starch during extrusion. Food Chem. 2021, 368, 130804. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, Y.; Qu, Y.; Guo, X.; Yang, X.; Cui, X.; Wang, C. Structural characterization of a novel polysaccharide from Panax notoginseng residue and its immunomodulatory activity on bone marrow dendritic cells. Int. J. Biol. Macromol. 2020, 161, 797–809. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Wang, F.; Xu, J.; Tang, X.; Li, N. Structural characterization and antioxidant activity of polysaccharide from ginger. Int. J. Biol. Macromol. 2018, 111, 862–869. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Li, G.; Yao, W.; Yang, W. The Gut–Organ-Axis Concept: Advances the Application of Gut-on-Chip Technology. Int. J. Mol. Sci. 2023, 24, 4089. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, D.; Hu, Y.; Huang, X.; Wang, J. Sulfated modification of epimedium polysaccharide and effects of the modifiers on cellular infectivity of IBDV. Carbohydr. Polym. 2008, 71, 180–186. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Liu, Y.; Si, C.-L.; Yuan, J.; Lv, Q.; Li, Y.-Y.; Dong, G.-L.; Liu, A.-J.; Zhang, Y.-M. Sulfated modification of the polysaccharide from Cordyceps_gunnii mycelia and its biological activities. Carbohydr. Polym. 2013, 92, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, Q.; Duan, X.; Tang, T.; Ke, Y.; Zhang, L.; Li, C.; Liu, A.; Su, Z.; Hu, B. Antioxidant and anticoagulant activities of mycelia polysaccharides from Catathelasma ventricosum after sulfated modification. Ind. Crop. Prod. 2018, 112, 53–60. [Google Scholar] [CrossRef]
- Vogl, H.; Paper, D.; Franz, G. Preparation of a sulfated linear (1→4)-β-d-galactan with variable degrees of sulfation. Carbohydr. Polym. 2000, 41, 185–190. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Chen, Z. Sulfated modification of the polysaccharides obtained from defatted rice bran and their antitumor activities. Int. J. Biol. Macromol. 2009, 44, 211–214. [Google Scholar] [CrossRef]
- Alban, S.; Schauerte, A.; Franz, G. Anticoagulant sulfated polysaccharides: Part I. Synthesis and structure–activity relationships of new pullulan sulfates. Carbohydr. Polym. 2002, 47, 267–276. [Google Scholar] [CrossRef]
- Chen, T.; Li, B.; Li, Y.; Zhao, C.; Shen, J.; Zhang, H. Catalytic synthesis and antitumor activities of sulfated polysaccharide from Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2011, 83, 554–560. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Ling, Y.; Fan, W.; Wang, Y.; Yin, H. Structural Determination and Antioxidant Activity of a Polysaccharide from the Fruiting Bodies of Cultured Cordyceps sinensis. Am. J. Chin. Med. 2009, 37, 977–989. [Google Scholar] [CrossRef]
- Hayat, K.; Zhang, X.; Chen, H.; Xia, S.; Jia, C.; Zhong, F. Liberation and separation of phenolic compounds from citrus mandarin peels by microwave heating and its effect on antioxidant activity. Sep. Purif. Technol. 2010, 73, 371–376. [Google Scholar] [CrossRef]
- Li, X. Improved Pyrogallol Autoxidation Method: A Reliable and Cheap Superoxide-Scavenging Assay Suitable for All Antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef]
- Lv, Q.-Q.; Cao, J.-J.; Liu, R.; Chen, H.-Q. Structural characterization, α-amylase and α-glucosidase inhibitory activities of polysaccharides from wheat bran. Food Chem. 2021, 341, 128218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr. Polym. 2016, 144, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Huang, G. Preparation and antioxidant activities of cuaurbit polysaccharide. Int. J. Biol. Macromol. 2018, 117, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.P.; Shapiro, R.H.; Albersheim, P. Quantitative analysis by various g.l.c. response-factor theories for partially methylated and partially ethylated alditol acetates. Carbohydr. Res. 1975, 40, 217–225. [Google Scholar] [CrossRef]
- Cao, W.; Li, X.-Q.; Liu, L.; Yang, T.-H.; Li, C.; Fan, H.-T.; Jia, M.; Lu, Z.-G.; Mei, Q.-B. Structure of an anti-tumor polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr. Polym. 2006, 66, 149–159. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Wang, D.; Wu, N.; Wang, K.; Zhang, Y. Preparation, chemical structure and α-glucosidase inhibitory activity of sulfated polysaccharide from Grifola frondosa. J. Funct. Foods 2022, 98, 105289. [Google Scholar] [CrossRef]
- De Moura, F.A.; Macagnan, F.T.; da Silva, L.P. Oligosaccharide production by hydrolysis of polysaccharides: A review. Int. J. Food Sci. Technol. 2015, 50, 275–281. [Google Scholar] [CrossRef]
- Xiao, H.; Fu, X.; Cao, C.; Li, C.; Chen, C.; Huang, Q. Sulfated modification, characterization, antioxidant and hypoglycemic activities of polysaccharides from Sargassum pallidum. Int. J. Biol. Macromol. 2019, 121, 407–414. [Google Scholar] [CrossRef]
- Ghosh, P.; Adhikari, U.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. In vitro anti-herpetic activity of sulfated polysaccharide fractions from Caulerpa racemosa. Phytochemistry 2004, 65, 3151–3157. [Google Scholar] [CrossRef]
- Jing, Y.; Zhu, J.; Liu, T.; Bi, S.; Hu, X.; Chen, Z.; Song, L.; Lv, W.; Yu, R. Structural Characterization and Biological Activities of a Novel Polysaccharide from Cultured Cordyceps militaris and Its Sulfated Derivative. J. Agric. Food Chem. 2015, 63, 3464–3471. [Google Scholar] [CrossRef]
- Huang, L.; Huang, M.; Shen, M.; Wen, P.; Wu, T.; Hong, Y.; Yu, Q.; Chen, Y.; Xie, J. Sulfated modification enhanced the antioxidant activity of Mesona chinensis Benth polysaccharide and its protective effect on cellular oxidative stress. Int. J. Biol. Macromol. 2019, 136, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-J.; Xie, J.-H.; Kan, L.-J.; Wang, J.-Q.; Shen, M.-Y.; Li, W.-J.; Nie, S.-P.; Xie, M.-Y. Sulfated polysaccharides from Cyclocarya paliurus reduce H2O2-induced oxidative stress in RAW264.7 cells. Int. J. Biol. Macromol. 2015, 80, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Zhang, Y.; Zhang, N.; Fu, L. Chemical modification and antioxidant activities of polysaccharide from mushroom Inonotus obliquus. Carbohydr. Polym. 2012, 89, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Govindan, S.; Ramani, P. Sulfated modification, characterization and bioactivities of an acidic polysaccharide fraction from an edible mushroom Pleurotus eous (Berk.) Sacc. Heliyon 2021, 7, e05964. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fu, J.; Yin, X.; Qu, C.; Yang, C.; He, H.; Ni, J. Induction of Apoptosis in HepaRG Cell Line by Aloe-Emodin through Generation of Reactive Oxygen Species and the Mitochondrial Pathway. Cell. Physiol. Biochem. 2017, 42, 685–696. [Google Scholar] [CrossRef]
- Kong, F.; Li, F.-E.; He, Z.; Jiang, Y.; Hao, R.; Sun, X.; Tong, H. Anti-tumor and macrophage activation induced by alkali-extracted polysaccharide from Pleurotus ostreatus. Int. J. Biol. Macromol. 2014, 69, 561–566. [Google Scholar] [CrossRef]
- Sahu, A.N.; Shreya, S.; Jain, S.K.; Guru, S.K. Anti-cancer potential of Pleurotus mushroom: Detailed insight on the potential bioactive molecules, Invitro-Invivo studies, and formulation. Lett. Drug Des. Discov. 2023, 20, 439–456. [Google Scholar] [CrossRef]
- Nataraj, A.; Govindan, S.; Rajendran, A.; Ramani, P.; Subbaiah, K.A.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M. Effects of Carboxymethyl Modification on the Acidic Polysaccharides from Calocybe indica: Physicochemical Properties, Antioxidant, Antitumor and Anticoagulant Activities. Antioxidants 2023, 12, 105. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Acetylation and carboxymethylation of the polysaccharide from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef]









| RT | Methylated Sugars | Mass Fragments (m/z) | Mole Ratio | LINKAGES |
|---|---|---|---|---|
| 24.396 | 1,5-Di-O-acetyl-1-deuterio-2,3,4,6-tetra-O-methyl-D-glucitol | 43, 71, 87, 101, 117, 129, 145, 161, 205 | 0.486 | Glcp-(1→ |
| 30.305 | 1,4,5-Tri-O-acetyl-1-deuterio-2,3,6-tri-O-methyl-D-glucitol | 43, 87, 99, 101, 113, 117, 129, 131, 161, 173, 233 | 0.359 | →4)-Glcp-(1→ |
| 31.340 | 1,5,6-Tri-O-acetyl-1-deuterio-2,3,4-tri-O-methyl-D-glucitol | 43, 87, 99, 101, 117, 129, 161, 189, 233 | 0.060 | →6-Glcp-(1→ |
| 37.506 | 1,4,5,6-Tetra-O-acetyl-1-deuterio-2,3-di-O-methyl-D-glucitol | 43, 71, 85, 87, 99, 101, 117, 127, 159, 161, 201 | 0.095 | →4,6)-Glcp-(1→ |
| Group | Early Apoptosis Rate (%) | Late Apoptosis Rate (%) | Total Apoptosis Rate (%) |
|---|---|---|---|
| NC | 10.1 ± 1.75 | 2.44 ± 1.83 | 12.54 ± 0.98 |
| 5-FU (200 µg/mL) | 57.5 ± 3.64 | 2.15 ± 1.56 | 58.36 ± 5.83 *** |
| SGP-1 (100 µg/mL) | 20.3 ± 2.67 | 1.82 ± 1.08 | 22.12 ± 2.51 ** |
| SGP-1 (200 µg/mL) | 31.0 ± 3.05 | 3.55 ± 1.66 | 34.55 ± 2.67 *** |
| SGP-1 (400 µg/mL) | 33.2 ± 3.19 | 2.21 ± 1.73 | 35.41 ± 3.13 *** |
| S-SGP (100 µg/mL) | 35.2 ± 4.16 | 3.07 ± 1.71 | 38.27 ± 3.11 *** |
| S-SGP (200 µg/mL) | 38.5 ± 3.26 | 1.07 ± 1.05 | 59.85 ± 3.98 *** |
| S-SGP (400 µg/mL) | 63.5 ± 4.25 | 1.68 ± 1.50 | 65.18 ± 4.12 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, P.; Wang, M.; Guo, Y.; Long, H.; Wang, Z.; Cui, D.; Yao, W.; Yang, W.; Chen, F.; Xie, J. Structure Characterization, In Vitro Antioxidant and Anti-Tumor Activity of Sulfated Polysaccharide from Siraitia grosvenorii. Foods 2023, 12, 2133. https://doi.org/10.3390/foods12112133
Gong P, Wang M, Guo Y, Long H, Wang Z, Cui D, Yao W, Yang W, Chen F, Xie J. Structure Characterization, In Vitro Antioxidant and Anti-Tumor Activity of Sulfated Polysaccharide from Siraitia grosvenorii. Foods. 2023; 12(11):2133. https://doi.org/10.3390/foods12112133
Chicago/Turabian StyleGong, Pin, Mengrao Wang, Yuxi Guo, Hui Long, Zhineng Wang, Dandan Cui, Wenbo Yao, Wenjuan Yang, Fuxin Chen, and Jianwu Xie. 2023. "Structure Characterization, In Vitro Antioxidant and Anti-Tumor Activity of Sulfated Polysaccharide from Siraitia grosvenorii" Foods 12, no. 11: 2133. https://doi.org/10.3390/foods12112133