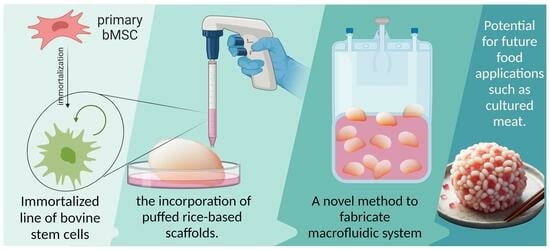
Cultivation of Bovine Mesenchymal Stem Cells on Plant-Based Scaffolds in a Macrofluidic Single-Use Bioreactor for Cultured Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Macrofluidic Devices
2.1.1. Laser Fabrication of Macrofluidic Devices from Thermoplastic Film
2.1.2. Fabrication of MSUB
2.1.3. Air Pump
2.1.4. Macrofluidic Prototyping for Plant-Based Scaffolds
2.1.5. Scaffold Sterilization
2.2. Cell Culture
2.2.1. Bovine MSC Immortalization
2.2.2. Expression of MSC Surface Markers
2.2.3. Seeding Cells on Rice Puff Scaffolds
2.2.4. Cellular Metabolic Activity
2.3. Microscopy
2.3.1. Fluorescence Microscopy
2.3.2. Scanning Electron Microscopy
3. Results
3.1. Rice-Based Scaffold Microscopy, Water Absorption, and pH Assay
3.2. Scanning Electron Microscopy (SEM)
3.3. Cell Line Properties in 2D
Proliferation Assay in Wells and in the MSUB
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAPE | Polyethylene–polyamide |
| MSUB | Macrofluidic single-use bioreactor |
References
- Levi, S.; Yen, F.; Baruch, L.; Machluf, M. Scaffolding technologies for the engineering of cultured meat: Towards a safe, sustainable, and scalable production. Trends Food Sci. Technol. 2022, 126, 13–25. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J. Three-dimensional scaffolds, materials, and fabrication for cultured meat applications: A scoping review and future direction. Food Hydrocoll. 2024, 152, 109881. [Google Scholar] [CrossRef]
- Bomkamp, C.; Skaalure, S.; Fernando, G.; Ben-Arye, T.; Swartz, E.; Specht, E. Scaffolding biomaterials for 3D cultivated meat: Prospects and challenges. Adv. Sci. 2022, 9, 2102908. [Google Scholar] [CrossRef]
- Seah, J.; Singh, S.; Tan, L.; Choudhury, D. Scaffolds for the manufacture of cultured meat. Crit. Rev. Biotechnol. 2022, 42, 311–323. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Han, S. Technological and structural aspects of scaffold manufacturing for cultured meat: Recent advances, challenges, and opportunities. Crit. Rev. Food Sci. Nutr. 2023, 63, 585–612. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, X.; Li, X.; Du, G.; Zhou, J.; Chen, J. Challenges and possibilities for bio-manufacturing cultured meat. Trends Food Sci. Technol. 2020, 97, 443–450. [Google Scholar] [CrossRef]
- Modulevsky, D.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A. Apple derived cellulose scaffolds for 3D mammalian cell culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- Xiang, N.; Yuen Jr, J.S.; Stout, A.J.; Rubio, N.R.; Chen, Y.; Kaplan, D.L. 3D porous scaffolds from wheat glutenin for cultured meat applications. Biomaterials 2022, 285, 121543. [Google Scholar] [CrossRef]
- Bar-Shai, N.; Sharabani-Yosef, O.; Zollmann, M.; Lesman, A.; Golberg, A. Seaweed cellulose scaffolds derived from green macroalgae for tissue engineering. Sci. Rep. 2021, 11, 11843. [Google Scholar] [CrossRef]
- Park, S.; Lee, M.; Jung, S.; Lee, H.; Choi, B.; Choi, M.; Lee, J.; Yoo, K.; Han, D.; Lee, S.; et al. Rice grains integrated with animal cells: A shortcut to a sustainable food system. Matter 2024, 7, 1292–1313. [Google Scholar] [CrossRef]
- De Wilde, D.; Dreher, T.; Zahnow, C.; Husemann, U.; Greller, G.; Adams, T.; Fenge, C. Superior scalability of single-use bioreactors. Innov. Cell Cult. 2014, 14, 14–19. [Google Scholar]
- Kurt, T.; Höing, T.; Oosterhuis, N. The Potential Application of Single-Use Bioreactors in Cultured Meat Production. Chem. Ing. Tech. 2022, 94, 2026–2030. [Google Scholar] [CrossRef]
- Bodiou, V.; Moutsatsou, P.; Post, M. Microcarriers for upscaling cultured meat production. Front. Nutr. 2020, 7, 10. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A. Selecting high amylose rice variety for puffing: A correlation between physicochemical parameters and sensory preferences. Meas. Food 2022, 5, 100021. [Google Scholar] [CrossRef]
- Jia, L.; Huang, R.; Wang, S.; Dong, Y.; Lv, J.; Zhong, W.; Yan, F. Effects of Explosion Puffing on the Composition, Structure, and Functional Characteristics of Starch and Protein in Grains. ACS Food Sci. Technol. 2021, 1, 1869–1879. [Google Scholar] [CrossRef]
- Bagchi, T.; Chattopadhyay, K.; Sarkar, S.; Sanghamitra, P.; Kumar, A.; Basak, N.; Sivashankari, M.; Priyadarsini, S.; Pathak, H. Rice Products and their Nutritional Status. Res. Bull. 2020, 23. [Google Scholar]
- Pashkuleva, I.; López-Pérez, P.; Azevedo, H.; Reis, R. Highly porous and interconnected starch-based scaffolds: Production, characterization and surface modification. Mater. Sci. Eng. C 2010, 30, 981–989. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.; Tian, L.; Shamirzaei-Jeshvaghani, E.; Dehghani, L.; Ramakrishna, S. Structural properties of scaffolds: Crucial parameters towards stem cells differentiation. World J. Stem Cells 2015, 7, 728. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar] [CrossRef]
- Kim, C.; Khil, M.; Kim, H.; Lee, H.; Jahng, K. An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J. Biomed. Mater. Res. Part Appl. Biomater. 2006, 78, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Horbett, T.; Schway, M.; Ratner, B. Hydrophilic-hydrophobic copolymers as cell substrates: Effect on 3T3 cell growth rates. J. Colloid Interface Sci. 1985, 104, 28–39. [Google Scholar] [CrossRef]
- Sbardelotto, P.; Balbinot-Alfaro, E.; da Rocha, M.; Alfaro, A. Natural alternatives for processed meat: Legislation, markets, consumers, opportunities and challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 10303–10318. [Google Scholar] [CrossRef] [PubMed]
- India, E. Puffed Rice Suppliers in India. Available online: https://www.exportersindia.com/indian-suppliers/puffed-rice.htm (accessed on 11 February 2024).
- IndiaMART. Puffed Rice Suppliers on IndiaMART. Available online: https://dir.indiamart.com/impcat/puffed-rice.htm (accessed on 11 February 2024).
- Chen, L.; Guttieres, D.; Koenigsberg, A.; Barone, P.; Sinskey, A.; Springs, S. Large-scale cultured meat production: Trends, challenges and promising biomanufacturing technologies. Biomaterials 2022, 280, 121274. [Google Scholar] [CrossRef] [PubMed]
- Djisalov, M.; Knežić, T.; Podunavac, I.; Živojević, K.; Radonic, V.; Knežević, N.; Bobrinetskiy, I.; Gadjanski, I. Cultivating multidisciplinarity: Manufacturing and sensing challenges in cultured meat production. Biology 2021, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Garrison, G.; Biermacher, J.; Brorsen, B. How much will large-scale production of cell-cultured meat cost? J. Agric. Food Res. 2022, 10, 100358. [Google Scholar] [CrossRef]
- Tuomisto, H.; Allan, S.; Ellis, M. Prospective life cycle assessment of a bioprocess design for cultured meat production in hollow fiber bioreactors. Sci. Total Environ. 2022, 851, 158051. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Selvaganapathy, P.; Geng, F. Advancing our understanding of bioreactors for industrial-sized cell culture: Health care and cellular agriculture implications. Am. J. -Physiol.-Cell Physiol. 2023, 325, C580–C591. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.; De Bank, P.; Ellis, M. Bioprocess design considerations for cultured meat production with a focus on the expansion bioreactor. Front. Sustain. Food Syst. 2019, 3, 44. [Google Scholar] [CrossRef]
- Thangadurai, M.; Srinivasan, S.; Sekar, M.; Sethuraman, S.; Sundaramurthi, D. Emerging perspectives on 3D printed bioreactors for clinical translation of engineered and bioprinted tissue constructs. J. Mater. Chem. B 2023, 12, 350–381. [Google Scholar] [CrossRef]
- Balakrishnan, H.; Doeven, E.; Merenda, A.; Dumée, L.; Guijt, R. 3D printing for the integration of porous materials into miniaturised fluidic devices: A review. Anal. Chim. Acta 2021, 1185, 338796. [Google Scholar] [CrossRef] [PubMed]
- Bader, C.; Patrick, W.; Kolb, D.; Hays, S.; Keating, S.; Sharma, S.; Dikovsky, D.; Belocon, B.; Weaver, J.; Silver, P.; et al. Grown, printed, and biologically augmented: An additively manufactured microfluidic wearable, functionally templated for synthetic microbes. 3D Print. Addit. Manuf. 2016, 3, 79–89. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Dikshit, V.; Zhang, Y. 3D-printed bioreactors for in vitro modeling and analysis. Int. J. Bioprinting 2020, 6, 267. [Google Scholar] [CrossRef] [PubMed]
- Merkel, M.; Noll, P.; Lilge, L.; Hausmann, R.; Henkel, M. Design and evaluation of a 3D-printed, lab-scale perfusion bioreactor for novel biotechnological applications. Biotechnol. J. 2023, 18, 2200554. [Google Scholar] [CrossRef] [PubMed]
- Linz, G.; Rauer, S.; Kuhn, Y.; Wennemaring, S.; Siedler, L.; Singh, S.; Wessling, M. 3D-Printed Bioreactor with Integrated Impedance Spectroscopy for Cell Barrier Monitoring. Adv. Mater. Technol. 2021, 6, 2100009. [Google Scholar] [CrossRef]
- Ng, S.; Kurisawa, M. Integrating biomaterials and food biopolymers for cultured meat production. Acta Biomater. 2021, 124, 108–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, J.; Kennedy, K.; Yeager, K.; Bernhard, J.; Ng, J.; Zimmerman, B.; Robinson, S.; Durney, K.; Shaeffer, C.; et al. Tissue engineered autologous cartilage-bone grafts for temporomandibular joint regeneration. Sci. Transl. Med. 2020, 12, eabb6683. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.; Gili Sole, L.; Reid, G.; Milan, G.; Hutter, G.; Grapow, M.; Eckstein, F.; Isu, G.; Marsano, A. Upscaled Skeletal Muscle Engineered Tissue with In Vivo Vascularization and Innervation Potential. Bioengineering 2023, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Samandari, M.; Saeedinejad, F.; Quint, J.; Chuah, S.; Farzad, R.; Tamayol, A. Repurposing biomedical muscle tissue engineering for cellular agriculture: Challenges and opportunities. Trends Biotechnol. 2023, 41, 887–906. [Google Scholar] [CrossRef]
- Humbird, D. Scale-up economics for cultured meat. Biotechnol. Bioeng. 2021, 118, 3239–3250. [Google Scholar] [CrossRef]
- Tsao, C. Polymer microfluidics: Simple, low-cost fabrication process bridging academic lab research to commercialized production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Ou, J.; Wilbert, J.; Haben, A.; Mi, H.; Ishii, H. milliMorph–Fluid-Driven Thin Film Shape-Change Materials for Interaction Design. In Proceedings of the 32nd Annual ACM Symposium on User Interface Software and Technology, New Orleans, LA, USA, 20–23 October 2019; pp. 663–672. [Google Scholar]
- Abdelgawad, M.; Wheeler, A. Low-cost, rapid-prototyping of digital microfluidics devices. Microfluid. Nanofluidics 2008, 4, 349–355. [Google Scholar] [CrossRef]
- Perrone, E.; Cesaria, M.; Zizzari, A.; Bianco, M.; Ferrara, F.; Raia, L.; Guarino, V.; Cuscunà, M.; Mazzeo, M.; Gigli, G.; et al. Potential of 5% CO2-laser processing of quartz for fast prototyping of microfluidic reactors and templates for 3D cell assembly over large scale. Mater. Today Bio 2021, 12, 100163. [Google Scholar] [CrossRef]
- Saadat, M.; Taylor, M.; Hughes, A.; Hajiyavand, A. Rapid prototyping method for 3D PDMS microfluidic devices using a red femtosecond laser. Adv. Mech. Eng. 2020, 12, 1687814020982713. [Google Scholar] [CrossRef]
- Hosic, S.; Bindas, A.J.; Puzan, M.L.; Lake, W.; Soucy, J.R.; Zhou, F.; Koppes, R.A.; Breault, D.T.; Murthy, S.K.; Koppes, A.N. Rapid prototyping of multilayer microphysiological systems. Acs Biomater. Sci. Eng. 2020, 7, 2949–2963. [Google Scholar] [CrossRef] [PubMed]
- Hanga, M.P.; Ali, J.; Moutsatsou, P.; de la Raga, F.A.; Hewitt, C.J.; Nienow, A.; Wall, I. Bioprocess development for scalable production of cultivated meat. Biotechnol. Bioeng. 2020, 117, 3029–3039. [Google Scholar] [CrossRef]
- Kirsch, M.; Morales-Dalmau, J.; Lavrentieva, A. Cultivated meat manufacturing: Technology, trends, and challenges. Eng. Life Sci. 2023, 23, e2300227. [Google Scholar] [CrossRef] [PubMed]
- Klebe, R.J.; Lyn, S.; Magnuson, V.L.; Zardeneta, G. Cultivation of mammalian cells in heat-sealable pouches that are 458 permeable to carbon dioxide. Exp. Cell Res. 1990, 188, 316–319. [Google Scholar] [CrossRef]
- Shaegh, S.A.M.; Pourmand, A.; Nabavinia, M.; Avci, H.; Tamayol, A.; Mostafalu, P.; Ghavifekr, H.B.; Aghdam, E.N.; Dokmeci, M.R.; Khademhosseini, A.; et al. Rapid prototyping of whole-thermoplastic microfluidics with built-in microvalves using laser ablation and thermal fusion bonding. Sens. Actuators Chem. 2018, 255, 100–109. [Google Scholar] [CrossRef]
- Truckenmüller, R.; Giselbrecht, S.; van Blitterswijk, C.; Dambrowsky, N.; Gottwald, E.; Mappes, T.; Rolletschek, A.; Saile, V.; Trautmann, C.; Weibezahn, K.F.; et al. Flexible fluidic microchips based on thermoformed and locally modified thin polymer films. Lab A Chip 2008, 8, 1570–1579. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Lim, H.R.; Kim, Y.S.; Hoang, T.T.T.; Choi, J.; Jeong, G.J.; Kim, H.; Herbert, R.; Soltis, I.; et al. Large-scale smart bioreactor with fully integrated wireless multivariate sensors and electronics for long-term in situ monitoring of stem cell culture. Sci. Adv. 2024, 10, eadk6714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Griffin, M.; Cai, J.; Li, S.; Bulter, P.E.; Kalaskar, D.M. Bioreactors for tissue engineering: An update. Biochem. Eng. J. 2016, 109, 268–281. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Gumfekar, S.P.; Bhanvase, B.A.; Sonawane, S.H.; Pimplapure, M.S. A review on microreactors: Reactor fabrication, design, and cutting-edge applications. Chem. Eng. Sci. 2018, 189, 431–448. [Google Scholar] [CrossRef]
- Hammond, T.; Hammond, J. Optimized suspension culture: The rotating-wall vessel. American J. -Physiol.-Ren. Physiol. 2001, 281, F12–F25. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Harrison, S.T.; Tai, S.L. Advances in bioreactor systems for the production of biologicals in mammalian cells. ChemBioEng Rev. 2022, 9, 42–62. [Google Scholar] [CrossRef]
- Hoyle, H.; Smith, L.; Williams, R.; Przyborski, S. Applications of novel bioreactor technology to enhance the viability and function of cultured cells and tissues. Interface Focus 2020, 10, 20190090. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.K.; Cole, S.Y.; Acuna Sierra, V.; Bracamonte, J.H.; Toldo, S.; Soares, J.S. Evaluation of perfusion-driven cell seeding of small diameter engineered tissue vascular grafts with a custom-designed seed-and-culture bioreactor. PLoS ONE 2022, 17, e0269499. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.F.; Thanarak, J.; Pontin, M.; Green, N.H.; Damian, D.D. Design and development of a robotic bioreactor for in vitro tissue engineering. In Proceedings of the 2021 IEEE International Conference on Robotics and Automation (ICRA), Xi’an, China, 30 May–5 June 2021; pp. 12428–12434. [Google Scholar]
- Hoyle, H.; Stenger, C.; Przyborski, S. Design considerations of benchtop fluid flow bioreactors for bio-engineered tissue equivalents in vitro. Biomater. Biosyst. 2022, 8, 100063. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Kreidl, E.; Bertschinger, M.; Vetsch, P. Benchtop bioreactors in mammalian cell culture: Overview and guidelines. Bioreact. Stem Cell Biol. Methods Protoc. 2021, 2346, 1–15. [Google Scholar]
- Reina-Mahecha, A.; Beers, M.J.; van der Veen, H.C.; Zuhorn, I.S.; van Kooten, T.G.; Sharma, P.K. A Review of the Role of Bioreactors for iPSCs-Based Tissue-Engineered Articular Cartilage. Tissue Eng. Regen. Med. 2023, 20, 1041–1052. [Google Scholar] [CrossRef]
- Todros, S.; Spadoni, S.; Maghin, E.; Piccoli, M.; Pavan, P.G. A novel bioreactor for the mechanical stimulation of clinically relevant scaffolds for muscle tissue engineering purposes. Processes 2021, 9, 474. [Google Scholar] [CrossRef]
- Bender, R.J.; Askinas, C.; Vernice, N.A.; Dong, X.; Harris, J.; Shih, S.; Spector, J.A. Perfuse and Reuse: A Low-Cost Three-Dimensional-Printed Perfusion Bioreactor for Tissue Engineering. Tissue Eng. Part C Methods 2022, 28, 623–633. [Google Scholar] [CrossRef]
- Kulus, M.; Jankowski, M.; Kranc, W.; Golkar Narenji, A.; Farzaneh, M.; Dzięgiel, P.; Zabel, M.; Antosik, P.; Bukowska, D.; Mozdziak, P.; et al. Bioreactors, scaffolds and microcarriers and in vitro meat production—current obstacles and potential solutions. Front. Nutr. 2023, 10, 1225233. [Google Scholar] [CrossRef]
- Gome, G.; Fein, Y.; Waksberg, J.; Maayan, Y.; Grishko, A.; Wald, I.Y.; Zuckerman, O. My First Biolab: A System for Hands-On Biology Experiments. In Proceedings of the Extended Abstracts of the 2019 CHI Conference on Human Factors in Computing Systems, Glasgow, UK, 4–9 May 2019; pp. 1–6. [Google Scholar]
- Baksh, D.; Song, L.; Tuan, R.S. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J. Cell. Mol. Med. 2004, 8, 301–316. [Google Scholar] [CrossRef]
- Hill, A.B.T.; Bressan, F.F.; Murphy, B.D.; Garcia, J.M. Applications of mesenchymal stem cell technology in bovine species. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Shimoni, C.; Goldstein, M.; Ribarski-Chorev, I.; Schauten, I.; Nir, D.; Strauss, C.; Schlesinger, S. Heat shock alters mesenchymal stem cell identity and induces premature senescence. Front. Cell Dev. Biol. 2020, 8, 565970. [Google Scholar] [CrossRef]
- Addgene. Human Telomerase Gene hTERT. Available online: https://www.addgene.org/1773/ (accessed on 11 February 2024).
- Wongsa, J.; Uttapap, D.; Lamsal, B.P.; Rungsardthong, V. Effect of puffing conditions on physical properties and rehydration characteristic of instant rice product. Int. J. Food Sci. Technol. 2016, 51, 672–680. [Google Scholar] [CrossRef]
- Shinde, R.; Vinokur, Y.; Fallik, E.; Rodov, V. Effects of Genotype and Modified Atmosphere Packaging on the Quality of Fresh-Cut Melons. Foods 2024, 13, 256. [Google Scholar] [CrossRef]
- Huang, H.D.; Ren, P.G.; Zhong, G.J.; Olah, A.; Li, Z.M.; Baer, E.; Zhu, L. Promising strategies and new opportunities for high barrier polymer packaging films. Prog. Polym. Sci. 2023, 144, 101722. [Google Scholar] [CrossRef]
- Horsthuis, E. The Future of Plastic Packaging for the Fresh Food Industry. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2023. [Google Scholar]
- Fazakerley, D. Limited oxygen in standard cell culture alters metabolism and function in differentiated cells. EMBO J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, D.G. Automation in Food Manufacturing and Processing. In Springer Handbook of Automation; Springer: Berlin/Heidelberg, Germany, 2023; pp. 949–971. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gome, G.; Chak, B.; Tawil, S.; Shpatz, D.; Giron, J.; Brajzblat, I.; Weizman, C.; Grishko, A.; Schlesinger, S.; Shoseyov, O. Cultivation of Bovine Mesenchymal Stem Cells on Plant-Based Scaffolds in a Macrofluidic Single-Use Bioreactor for Cultured Meat. Foods 2024, 13, 1361. https://doi.org/10.3390/foods13091361
Gome G, Chak B, Tawil S, Shpatz D, Giron J, Brajzblat I, Weizman C, Grishko A, Schlesinger S, Shoseyov O. Cultivation of Bovine Mesenchymal Stem Cells on Plant-Based Scaffolds in a Macrofluidic Single-Use Bioreactor for Cultured Meat. Foods. 2024; 13(9):1361. https://doi.org/10.3390/foods13091361
Chicago/Turabian StyleGome, Gilad, Benyamin Chak, Shadi Tawil, Dafna Shpatz, Jonathan Giron, Ilan Brajzblat, Chen Weizman, Andrey Grishko, Sharon Schlesinger, and Oded Shoseyov. 2024. "Cultivation of Bovine Mesenchymal Stem Cells on Plant-Based Scaffolds in a Macrofluidic Single-Use Bioreactor for Cultured Meat" Foods 13, no. 9: 1361. https://doi.org/10.3390/foods13091361