Pompon Mum-like SiO2/C Nanospheres with High Performance as Anodes for Lithium-Ion Batteries
Abstract
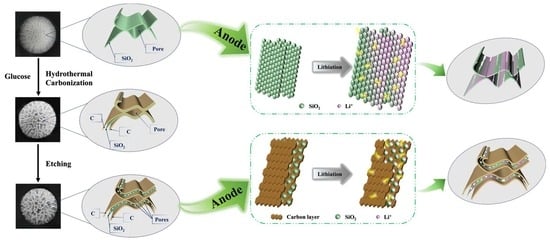
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pompon Mum-like SiO2/C Nanospheres
2.3. Materials Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Morphology and Structure of Composite Materials
3.2. Electrochemical Performances
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, R.; Kumar, H.; Kumar, G.; Sharma, S.; Aneja, R.; Sharma, A.K.; Kumar, R.; Kumar, P. Progress and challenges in electrochemical energy storage devices: Fabrication, electrode material, and economic aspects. Chem. Eng. J. 2023, 468, 143706. [Google Scholar] [CrossRef]
- Obrovac, M.; Christensen, L. Structural changes in silicon anodes during lithium insertion/extraction. Electrochem. Solid-State Lett. 2004, 7, A93. [Google Scholar] [CrossRef]
- Choi, S.; Wang, G. Advanced lithium-ion batteries for practical applications: Technology, development, and future perspectives. Adv. Mater. Technol. 2018, 3, 1700376. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.; Cheng, H.-M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Recent progress in advanced materials for lithium ion batteries. Materials 2013, 6, 156–183. [Google Scholar] [CrossRef] [PubMed]
- Balogun, M.; Qiu, W.; Luo, Y.; Meng, H.; Mai, W.; Onasanya, A.; Olaniyi, T.K. A review of the development of full cell lithium-ion batteries: The impact of nanostructured anode materials. Nano Res. 2016, 9, 2823–2851. [Google Scholar] [CrossRef]
- Du, C.; Zhao, Z.; Liu, H.; Song, F.; Chen, L.; Cheng, Y.; Guo, Z. The status of representative anode materials for lithium-ion batteries. Chem. Rec. 2023, 23, e202300004. [Google Scholar] [CrossRef] [PubMed]
- Maurin, G.; Bousquet, C.; Henn, F.; Bernier, P.; Almairac, R.; Simon, B. Electrochemical intercalation of lithium into multiwall carbon nanotubes. Chem. Phys. Lett. 1999, 312, 14–18. [Google Scholar] [CrossRef]
- Yin, Y.; Wan, L.; Guo, Y. Silicon-based nanomaterials for lithium-ion batteries. Chin. Sci. Bull. 2012, 57, 4104–4110. [Google Scholar] [CrossRef]
- Maresca, G.; Sankaran, A.; Santa Maria, L.J.; Ottaviani, M.; Fantini, S.; Ryan, K.M.; Brutti, S.; Appetecchi, G.B. Superior compatibility of silicon nanowire anodes in ionic liquid electrolytes. Energy Mater. 2024, 4, 400017. [Google Scholar] [CrossRef]
- Wang, X.; Niu, C.; Cui, L.; Qiao, F.; Wang, J.; Mai, L. Solution-catalyzed carbothermal reduction of argo-waste SiO2 enables low-temperature and fast synthesis of Si (Ⅱ)-C anode. Chem. Eng. J. 2023, 472, 145116. [Google Scholar]
- Liu, Z.; Wang, X.; Hu, J.; Meng, J.; Niu, C.; Liu, F.; Cui, L.; Yu, R.; Mai, L. High-capacity sub-nano divalent silicon from biosilicification. Adv. Energy Mater. 2023, 13, 2301715. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, R.; Feng, S.; Lin, Z.; Zhu, M. SiO–Sn2Fe@ C composites with uniformly distributed Sn2Fe nanoparticles as fast-charging anodes for lithium-ion batteries. eScience 2023, 3, 100080. [Google Scholar] [CrossRef]
- Tu, J.; Yuan, Y.; Zhan, P.; Jiao, H.; Wang, X.; Zhu, H.; Jiao, S. Straightforward approach toward SiO2 nanospheres and their superior lithium storage performance. J. Phys. Chem. C 2014, 118, 7357–7362. [Google Scholar] [CrossRef]
- Hu, G.; Sun, X.; Liu, H.; Xu, Y.; Liao, L.; Guo, D.; Liu, X.; Qin, A. Improvement of lithium storage performance of silica anode by using ketjen black as functional conductive agent. Nanomaterials 2022, 12, 692. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, B.; Fu, Z. Lithium electrochemistry of SiO2 thin film electrode for lithium-ion batteries. Appl. Surf. Sci. 2008, 254, 3774–3779. [Google Scholar] [CrossRef]
- Ozen, S.; Eroglu, O.; Karatepe, N. Electrochemically pre-lithiated SiO2@C nanocomposite anodes for improved performance in lithium-ion batteries. Nanotechnology 2023, 34, 485403. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Sun, H.; Wang, K.; Fan, C.-Y.; Zhang, L.-L.; Yang, F.-M.; Zhang, J.-P. Dual-porosity SiO2/C nanocomposite with enhanced lithium storage performance. J. Phys. Chem. C 2015, 119, 3495–3501. [Google Scholar] [CrossRef]
- Jiao, M.; Liu, K.; Shi, Z.; Wang, C. SiO2/Carbon composite microspheres with hollow core-shell structure as a high-stability electrode for lithium-ion batteries. ChemElectroChem 2017, 4, 542–549. [Google Scholar] [CrossRef]
- Chen, K.; Tan, Y.; Wang, K.; Niu, J.; Chen, Z.Y. High specific capacity of carbon coating lemon-like SiO2 hollow spheres for lithium-ion batteries. Electrochim. Acta 2022, 401, 139497. [Google Scholar] [CrossRef]
- Cao, X.; Chuan, X.; Massé, R.; Huang, D.; Li, S.; Cao, G. A three layer design with mesoporous silica encapsulated by a carbon core and shell for high energy lithium ion battery anodes. J. Mater. Chem. A 2015, 3, 22739–22749. [Google Scholar] [CrossRef]
- Huang, S.; Yang, D.; Zhang, W.; Qiu, X.; Li, Q.; Li, C. Dual-templated synthesis of mesoporous lignin-derived honeycomb-like porous carbon/SiO2 composites for high-performance Li-ion battery. Microporous Mesoporous Mater. 2021, 317, 111004. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, W.; Yu, P.; Pan, N.; Hu, H.; Zheng, M.; Xiao, Y.; Liu, Y.; Liang, Y. Facile synthesis of core-shell structured SiO2@carbon composite nanorods for high-performance lithium-ion batteries. Nanomaterials 2020, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, Q.; Ma, W.; Li, H. Interfacial engineering of polyhedral carbon@hollowed carbon@SiO2 nanobox with tunable structure for enhanced lithium ion battery. Appl. Surf. Sci. 2021, 538, 148039. [Google Scholar] [CrossRef]
- Sui, D.; Yao, M.; Si, L.; Yan, K.; Shi, J.; Wang, J.; Xu, C.C.; Zhang, Y. Biomass-derived carbon coated SiO2 nanotubes as superior anode for lithium-ion batteries. Carbon 2023, 205, 510–518. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Liu, H.; Liu, Z. SiO2@C hollow sphere anodes for lithium-ion batteries. J. Mater. Sci. Technol. 2017, 33, 239–245. [Google Scholar] [CrossRef]
- Maity, A.; Belgamwar, R.; Polshettiwar, V. Facile synthesis to tune size, textural properties and fiber density of dendritic fibrous nanosilica for applications in catalysis and CO2 capture. Nat. Protoc. 2019, 14, 2177–2204. [Google Scholar] [CrossRef]
- Zhu, J.; Yao, C.; Maity, A.; Xu, J.; Zhan, T.; Liu, W.; Sun, M.; Wang, S.; Polshettiwar, V.; Tan, H. Nitrogen doped carbon spheres with wrinkled cages for the selective oxidation of 5-hydroxymethylfurfural to 5-formyl-2-furancarboxylic acid. Chem. Commun. 2021, 57, 2005–2008. [Google Scholar] [CrossRef]
- Haskouri, J.; José, M.; David, O.; Fernández, L.; Latorre, J.; Guillem, C.; Beltrán, A.; Beltrán, D.; Amorós, P. Nanoparticulated silicas with bimodal porosity: Chemical control of the pore sizes. Inorg. Chem. 2008, 47, 8267–8277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Das, S.; Xing, G.; Fayon, P.; Heasman, P.; Jay, M.; Bailey, S.; Lambert, C.; Yamada, H.; Wakihara, T.; et al. A 3D organically synthesized porous carbon material for lithium-ion batteries. Angew. Chem. Int. Ed. 2018, 57, 11952–11956. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, X.; Hao, X.; Xu, Y.; Tang, S.; Zhang, K.; Qin, A. Few layers 2D MoS2/tubular sisal fiber-derived carbon composite: Enhanced cycling performance as anode material for sodium-ion batteries. J. Energy Storage 2023, 67, 107463. [Google Scholar] [CrossRef]
- Yu, R.; Jiang, R.; Zhou, Z. Yolk-shell SiO2 wrapped by reduced graphene oxide for high performance lithium-ion battery anode. J. Alloys Compd. 2023, 937, 168324. [Google Scholar] [CrossRef]
- Chen, K.; Tan, Y.; Gao, Y. Facile synthesis of pomegranate-like structured SiOx composite spheres with internal carbon conductive network for lithium-ion batteries. J. Power Sources 2023, 581, 233493. [Google Scholar] [CrossRef]
- Shi, C.; Chen, J.; Guo, T.; Luo, G.; Shi, H.; Shi, Z.; Qin, G.; Zhang, L.; He, X. Controllable preparation to boost high performance of nanotubular SiO2@C as anode materials for lithium-ion batteries. Batteries 2023, 9, 107. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Wang, Y.; Zong, P.; Zhang, L.; Zhang, Z.; Gu, X.; Qiao, Y.; Lu, G.; Tian, Y. Adjusting ash content of char to enhance lithium storage performance of rice husk-based SiO2/C. J. Alloys Compd. 2021, 854, 156986. [Google Scholar]
- Liu, H.; Liu, X.; Liu, Z.; Tao, J.; Dai, X.; Yang, Q.; Xua, J.; Shan, Z. Graphite@ silicon embedded in a carbon conformally coated tiny SiO2 nanoparticle matrix for high-performance lithium-ion batteries. Inorg. Chem. Front. 2021, 8, 4395–4406. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, N.; Shi, C.; Liu, E.; He, C.; He, F.; Ma, L. In-situ grown CNTs modified SiO2/C composites as anode with improved cycling stability and rate capability for lithium storage. Appl. Surf. Sci. 2018, 433, 428–436. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, K.; He, W.; Liu, Y.; Guo, S. SiO2@ graphite composite generated from sewage sludge as anode material for lithium ion batteries. Int. J. Electrochem. Sci. 2017, 12, 10221–10229. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Z.; Chen, L. Amorphous SiO2 in tunnel-structured mesoporous carbon and its anode performance in Li-ion batteries. Mater. Des. 2016, 111, 616–621. [Google Scholar] [CrossRef]
- Belgibayeva, A.; Taniguchi, I. Synthesis and characterization of SiO2/C composite nanofibers as free-standing anode materials for Li-ion batteries. Electrochim. Acta 2019, 328, 135101. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Q.; Zhang, J.; Xie, X.; Xia, B. Green synthesis of dual carbon conductive network-encapsulated hollow SiOx spheres for superior lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 19959–19967. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Wang, F.; Han, F.; He, Y.; Zhang, F.; Liu, J. Improving the lithium storage performance of micro-sized SiOx particles by uniform carbon interphase encapsulation and suitable SiO2 buffer component. Electrochim. Acta 2021, 385, 138431. [Google Scholar] [CrossRef]
- Luo, K.; Wu, K.; Hou, Q.; Zhang, W.; Jiang, T.; Wang, X.; Liu, X.; Liu, W. Spider-web-inspired cellulose nanofibrils networking polyaniline-encapsulated silica nanoparticles as anode material of lithium-ion batteries. Carbohydr. Polym. 2022, 277, 118833. [Google Scholar] [CrossRef] [PubMed]
- Dees, D.; Kawauchi, S.; Abraham, D.; Prakash, J. Analysis of the galvanostatic intermittent titration technique (GITT) as applied to a lithium-ion porous electrode. J. Power Sources 2009, 189, 263–268. [Google Scholar] [CrossRef]
- Hu, G.; Liu, H.; Luo, Y.; Zhang, K.; Guo, D.; Liu, X.; Qin, A. Two-dimensional sandwich-like silica@ carbon@ silica nanosheets for superior lithium storage. Mater. Lett. 2022, 308, 131288. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, S.; Kang, Z.; Jiao, S. Facile synthesis of SiO2/C composite and its application as anode material for lithium ion batde. Electrochemistry 2015, 83, 421–424. [Google Scholar] [CrossRef]
- Liang, C.; Chen, Y.; Xu, H.; Xia, Y.; Hou, X.; Gan, Y.; Ma, X.; Tao, X.; Huang, H.; Zhang, J.; et al. Embedding submicron SiO2 into porous carbon as advanced lithium-ion batteries anode with ultralong cycle life and excellent rate capability. J. Taiwan Inst. Chem. Eng. 2019, 95, 227–233. [Google Scholar] [CrossRef]
- Ali, S.; Jaffer, S.; Maitlo, I.; Shehzad, F.K.; Wang, Q.; Ali, S.; Akram, M.Y.; He, Y.; Nie, J. Photo cured 3D porous silica-carbon (SiO2-C) membrane as anode material for high performance rechargeable Li-ion batteries. J. Alloys Compd. 2020, 812, 152127. [Google Scholar] [CrossRef]
- Ren, Y.R.; Yang, B.; Wei, H.M.; Ding, J. Electrospun SiO2/C composite fibers as durable anode materials for lithium ion batteries. Solid State Ion. 2016, 292, 27–31. [Google Scholar] [CrossRef]












| Samples | SBET (m2 g−1) | Vt (cm3 g−1) | (nm) |
|---|---|---|---|
| DFNS | 223 | 0.33 | 5.43 |
| C/DFNS-50 | 127 | 0.06 | 4.18 |
| C/DFNS-10 | 573 | 0.46 | 3.47 |
| C/DFNS-6 | 672 | 0.64 | 3.52 |
| C/DFNS-1 | 650 | 0.60 | 3.51 |
| Materials | Morphology | Current Density (A g−1) | Reversible Capacity (mAh g−1)/Cycles | Ref. |
|---|---|---|---|---|
| DMSNs/C | Spheres | 0.1 | 635.7/200 | [20] |
| SiO2/po-C@C | Hollow core–shell microspheres | 0.1 | 669.8/100 | [21] |
| L-HSiO2@CN | Hollow mesoporous nanospheres | 0.1 1.0 | 841.9/248 786/500 | [22] |
| C-mcms | Triple spheres | 0.5 | 1055/150 | [23] |
| LHC/SiO2-21 | Honeycomb-like porous spheres | 0.1 5.0 | 1109/100 230/1000 | [24] |
| SiO2@CCNRs-2 | Core–shell nanorods | 0.1 | 690/100 | [25] |
| PC@HC@SiO2 | Nano boxes | 0.1 1.0 | 723/150 321/800 | [23] |
| SNTs@c-PDLF | Hollow nanotubes | 0.1 1.0 | 911/300 549/800 | [27] |
| SiO2/C composite | Irregular polyhedrons | 0.186 | 533.4/100 | [48] |
| SiO2/C | Irregular particle shape | 0.1 1.0 | 620/150 534/1000 | [49] |
| SiO2/C | Porous irregular shape | 0.2 2.0 | 886/100 359/500 | [37] |
| SiO2@cPANI/cTOCNFs | 3D spider-web-like nanostructure | 0.1 1.0 | 1103/200 302/1000 | [45] |
| SiO2-C material | 3D porous membranes | 0.1 | 642/100 | [50] |
| SiO2/C fiber | Fibers | 0.05 | 465/50 | [51] |
| C/DFNS-6 | Pompon mum-like nanospheres | 0.2 1.0 | 986/200 529/300 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Luo, Y.; Li, X.; Wang, Y.; Lin, S.; Ding, W.; Guo, K.; Zhang, K.; Qin, A. Pompon Mum-like SiO2/C Nanospheres with High Performance as Anodes for Lithium-Ion Batteries. Batteries 2024, 10, 149. https://doi.org/10.3390/batteries10050149
Sun X, Luo Y, Li X, Wang Y, Lin S, Ding W, Guo K, Zhang K, Qin A. Pompon Mum-like SiO2/C Nanospheres with High Performance as Anodes for Lithium-Ion Batteries. Batteries. 2024; 10(5):149. https://doi.org/10.3390/batteries10050149
Chicago/Turabian StyleSun, Xiaohui, Yuan Luo, Xuenuan Li, Yujie Wang, Shilong Lin, Weile Ding, Kailong Guo, Kaiyou Zhang, and Aimiao Qin. 2024. "Pompon Mum-like SiO2/C Nanospheres with High Performance as Anodes for Lithium-Ion Batteries" Batteries 10, no. 5: 149. https://doi.org/10.3390/batteries10050149