Minerals and the Menstrual Cycle: Impacts on Ovulation and Endometrial Health
Abstract
:1. Introduction
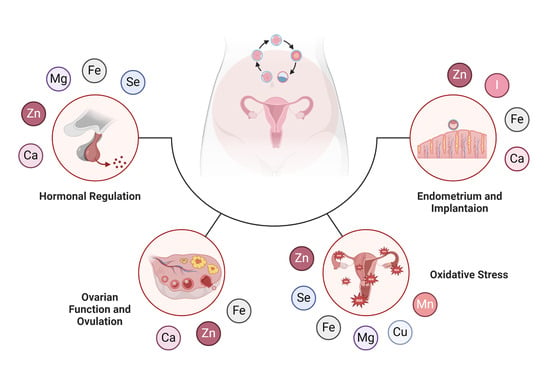
2. Hormonal Regulation and Fertility
2.1. Overview of Hormones and Fertility
2.2. Influence of Minerals on Hormonal Regulation
2.2.1. Zinc
2.2.2. Selenium
2.2.3. Iodine
2.2.4. Iron
2.2.5. Calcium
2.2.6. Magnesium
3. Ovarian Function and Ovulation
3.1. Overview of Ovarian Function
3.2. Influence of Minerals on Ovulation
3.2.1. Calcium
3.2.2. Zinc
3.2.3. Iron
4. Oxidative Stress and Fertility
4.1. Overview of Oxidative Stress and Its Influence on Fertility
4.2. Influence of Minerals on Oxidative Stress
4.2.1. Zinc
4.2.2. Selenium
4.2.3. Copper
4.2.4. Iron
4.2.5. Magnesium
4.2.6. Manganese
5. Endometrium and Embryo Implantation
5.1. Overview of Implantation and Endometrium in Fertility
5.2. Influence of Minerals on Implantation and Endometrium
5.2.1. Iron
5.2.2. Magnesium
5.2.3. Zinc
5.2.4. Calcium
5.2.5. Iodine
6. Conclusions
| Mineral | Hormonal Regulation | Ovarian Function and Ovulation | Oxidative Stress | Endometrium and Implantation |
|---|---|---|---|---|
| Zn | Insulin metabolism [105] Steroid synthesis [39,40] Hormone Balance [37] Regulation of LH and FSH [38] Ovulation [219] | Oocyte maturation [208,209], development [228], and quality [226] | Antioxidant properties [264,269], protect embryos from ROS [43,44], and modulate inflammation in endometriosis [281,368] | Cellular proliferation and differentiation in the endometrium [56], neutralizes alterations [279] and deficiency correlates with endometrial cysts and polyps [47,48] |
| Mg | Cofactor for the production and function of estrogen [163,164], stabilizing glucose metabolism [174] and disbalances are associated with PCOS [165,166,168] | Mg deficiency leads to oxidative stress [327,329,330]. Magnesium can preserve the quality of the oocyte [251,327], modulate the ovulation process [338], and contribute to the healthy functioning of the endometrium [337]. | Relax smooth muscle [54], influence retrograde menstruation [54,55], and reduce vascular endothelial growth factor, which may be beneficial in the treatment of gynecological conditions like endometriosis [57,58,59] | |
| Ca | Calcium influences the release of GnRH, and therefore the menstrual cycle [29,30] Necessary for ovulation [396] | Oocyte activation and zygotic development [31,32], oocyte activation and fusion with sperm [33,34,35] | - | Calcium is deposited at the site of embryo implantation and regulates endometrial receptivity and embryo implantation [45,46] |
| I | Thyroid function and the synthesis of thyroid hormones, also reproductive hormones [62]. Deficiency [397], resulting in anovulation, reduced fertility, and menstrual cycle disturbances [65,66] | - | - | Improving endometrial receptivity [380,381,382] and supporting embryo implantation through endometrial changes [383,384,386] |
| Se | Thyroid function and thus important for hormone regulation [60,61] Thyroid disorders can lead to disrupted menstrual cycles and anovulation [63,64] | - | Supporting Glutathione Peroxidase (GPx) [263,290,293] Efficient ovulation and protection of the ovaries from damage [41,42] | - |
| Fe | Hormone synthesis and regulation of estrogen and progesterone [18,19,20] Deficiency can cause hormonal imbalances and affect menstrual cycles [21,22,23,24,25] | Maturation of oocytes [232,233,234,235,236] and cellular division during oocyte maturation [237] Deficiency leads to anemia, compromises the blood flow to the ovaries, and affects the quality of oocytes and the regularity of ovulation [26,27,28] | Deficiency and overdose lead to oxidative stress [262,314,315] and both reduce oocyte quality [24,102,236,323] | Deficiency impacts endometrial conditions and its receptiveness and decreases embryo implantation [49,50,51,52,53] Ferritin overdose is in correlation with endometriosis [241,242,246] |
| Cu | - | Cofactor for Superoxide Dismutase [266] and modulates antioxidant systems [304,305,306,307] Protects oocytes from oxidative stress [307,308] and supports endothelial function; crucial for optimal blood flow to the uterus and ovaries [309,310,311,312,313] | - | |
| Mn | - | Cofactor for Superoxide Dismutase [341,342] and therefore supports oocyte quality and function [344,345] Overdose can contribute to oxidative stress and can damage oocyte and ovarian function [347,348,349] | - | |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Diet and Lifestyle in the Prevention of Ovulatory Disorder Infertility. Obstet. Gynecol. 2007, 110, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Chen, G.; Kunselman, A.R.; Schlaff, W.D.; Diamond, M.P.; Coutifaris, C.; Carson, S.A.; Steinkampf, M.P.; Carr, B.R.; McGovern, P.G.; et al. Smoking in Infertile Women with Polycystic Ovary Syndrome: Baseline Validation of Self-Report and Effects on Phenotype. Hum. Reprod. 2014, 29, 2680–2686. [Google Scholar] [CrossRef] [PubMed]
- Ethier, A.R.; McKinney, T.L.; Tottenham, L.S.; Gordon, J.L. The Effect of Reproductive Hormones on Women’s Daily Smoking across the Menstrual Cycle. Biol. Sex Differ. 2021, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Skoracka, K.; Ratajczak, A.E.; Rychter, A.M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Female Fertility and the Nutritional Approach: The Most Essential Aspects. Adv. Nutr. 2021, 12, 2372–2386. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Chavarro, J.E. Diet and Fertility: A Review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A. Cadmium Toxicity: Effects on Human Reproduction and Fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Mirnamniha, M.; Faroughi, F.; Tahmasbpour, E.; Ebrahimi, P.; Beigi Harchegani, A. An Overview on Role of Some Trace Elements in Human Reproductive Health, Sperm Function and Fertilization Process. Rev. Environ. Health 2019, 34, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Buhling, K.J.; Laakmann, E. The Effect of Micronutrient Supplements on Male Fertility. Curr. Opin. Obstet. Gynecol. 2014, 26, 199–209. [Google Scholar] [CrossRef]
- Almujaydil, M.S. The Role of Dietary Nutrients in Male Infertility: A Review. Life 2023, 13, 519. [Google Scholar] [CrossRef]
- Mora-Esteves, C.; Shin, D. Nutrient Supplementation: Improving Male Fertility Fourfold. Semin. Reprod. Med. 2013, 31, 293–300. [Google Scholar] [CrossRef]
- Dring, J.C.; Forma, A.; Chilimoniuk, Z.; Dobosz, M.; Teresiński, G.; Buszewicz, G.; Flieger, J.; Cywka, T.; Januszewski, J.; Baj, J. Essentiality of Trace Elements in Pregnancy, Fertility, and Gynecologic Cancers-A State-of-the-Art Review. Nutrients 2021, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Rezk, M.; Grasegger, L.; Brandstetter, N.; Pol-Edern, L.R.; Stelzl, P.; Oppelt, P.; Arbeithuber, B. Biomarker Screening in Preeclampsia: An RNA-Sequencing Approach Based on Data from Multiple Studies. J. Hypertens. 2022, 40, 2022–2036. [Google Scholar] [CrossRef] [PubMed]
- Grajecki, D.; Zyriax, B.-C.; Buhling, K.J. The Effect of Micronutrient Supplements on Female Fertility: A Systematic Review. Arch. Gynecol. Obstet. 2012, 285, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E.; Porteous, R.; Pape, J.-R.; Mora, J.M.; Hurst, P.R. Gonadotropin-Releasing Hormone Neuron Requirements for Puberty, Ovulation, and Fertility. Endocrinology 2008, 149, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.G. Gonadotropic Control of Ovarian Follicular Growth and Development. Mol. Cell. Endocrinol. 2001, 179, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Russell, D.L.; Ochsner, S.; Espey, L.L. Ovulation: New Dimensions and New Regulators of the Inflammatory-like Response. Annu. Rev. Physiol. 2002, 64, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, J.; Ghosh, D. Role of Progesterone on Peri-Implantation Stage Endometrium-Embryo Interaction in the Primate. Steroids 2000, 65, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Percy, L.; Mansour, D.; Fraser, I. Iron Deficiency and Iron Deficiency Anaemia in Women. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 40, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Hamza, I.; Bartnikas, T.B. Molecular Mechanisms of Iron and Heme Metabolism. Annu. Rev. Nutr. 2022, 42, 311–335. [Google Scholar] [CrossRef]
- Srole, D.N.; Ganz, T. Erythroferrone Structure, Function, and Physiology: Iron Homeostasis and beyond. J. Cell. Physiol. 2021, 236, 4888–4901. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; De-Regil, L.M.; Gomez Malave, H.; Flores-Urrutia, M.C.; Dowswell, T. Intermittent Oral Iron Supplementation during Pregnancy. Cochrane Database Syst. Rev. 2015, 2015, CD009997. [Google Scholar] [CrossRef] [PubMed]
- Petraglia, F.; Dolmans, M.M. Iron Deficiency Anemia: Impact on Women’s Reproductive Health. Fertil. Steril. 2022, 118, 605–606. [Google Scholar] [CrossRef]
- Mirza, F.G.; Abdul-Kadir, R.; Breymann, C.; Fraser, I.S.; Taher, A. Impact and Management of Iron Deficiency and Iron Deficiency Anemia in Women’s Health. Expert Rev. Hematol. 2018, 11, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Santini, V.; Braxs, C.; Shander, A. Iron Metabolism and Iron Deficiency Anemia in Women. Fertil. Steril. 2022, 118, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Li, Y.; Song, D.; Ding, J.; Mei, S.; Sun, S.; Cheng, W.; Yu, J.; Zhou, L.; Kuang, Y.; et al. Iron-Overloaded Follicular Fluid Increases the Risk of Endometriosis-Related Infertility by Triggering Granulosa Cell Ferroptosis and Oocyte Dysmaturity. Cell Death Dis. 2022, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Tonai, S.; Kawabata, A.; Nakanishi, T.; Lee, J.Y.; Okamoto, A.; Shimada, M.; Yamashita, Y. Iron Deficiency Induces Female Infertile in Order to Failure of Follicular Development in Mice. J. Reprod. Dev. 2020, 66, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.M. The Reproductive Ecology of Iron in Women. Am. J. Phys. Anthropol. 2016, 159, S172–S195. [Google Scholar] [CrossRef] [PubMed]
- Osungbade, K.O.; Oladunjoye, A.O. Preventive Treatments of Iron Deficiency Anaemia in Pregnancy: A Review of Their Effectiveness and Implications for Health System Strengthening. J. Pregnancy 2012, 2012, 454601. [Google Scholar] [CrossRef] [PubMed]
- Constantin, S.; Jasoni, C.; Romanò, N.; Lee, K.; Herbison, A.E. Understanding Calcium Homeostasis in Postnatal Gonadotropin-Releasing Hormone Neurons Using Cell-Specific Pericam Transgenics. Cell Calcium 2012, 51, 267–276. [Google Scholar] [CrossRef]
- Herbison, A.E. Control of Puberty Onset and Fertility by Gonadotropin-Releasing Hormone Neurons. Nat. Rev. Endocrinol. 2016, 12, 452–466. [Google Scholar] [CrossRef]
- Webb, S.E.; Miller, A.L. Calcium Signalling during Embryonic Development. Nat. Rev. Mol. Cell Biol. 2003, 4, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M. Calcium at Fertilization and in Early Development. Physiol. Rev. 2006, 86, 25–88. [Google Scholar] [CrossRef]
- Machaty, Z. Signal Transduction in Mammalian Oocytes during Fertilization. Cell Tissue Res. 2016, 363, 169–183. [Google Scholar] [CrossRef]
- Amireault, P.; Dubé, F. Intracellular cAMP and Calcium Signaling by Serotonin in Mouse Cumulus-Oocyte Complexes. Mol. Pharmacol. 2005, 68, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Michopoulos, A.; Daponte, A.; Chatzimeletiou, K.; Simopoulou, M.; Messini, C.I.; Polyzos, N.P.; Vassiou, K.; Dafopoulos, K.; Goulis, D.G. Artificial Oocyte Activation: Physiological, Pathophysiological and Ethical Aspects. Syst. Biol. Reprod. Med. 2019, 65, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, O.; Weiss, J.M.; Diedrich, K. Gonadotrophin-Releasing Hormone (GnRH) and GnRH Agonists: Mechanisms of Action. Reprod. Biomed. Online 2002, 5 (Suppl. S1), 1–7. [Google Scholar] [CrossRef]
- Nasiadek, M.; Stragierowicz, J.; Klimczak, M.; Kilanowicz, A. The Role of Zinc in Selected Female Reproductive System Disorders. Nutrients 2020, 12, 2464. [Google Scholar] [CrossRef]
- Bedwal, R.S.; Bahuguna, A. Zinc, Copper and Selenium in Reproduction. Experientia 1994, 50, 626–640. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Baltaci, S.B. Review: The Role of Zinc in the Endocrine System. Pak. J. Pharm. Sci. 2019, 32, 231–239. [Google Scholar]
- Te, L.; Liu, J.; Ma, J.; Wang, S. Correlation between Serum Zinc and Testosterone: A Systematic Review. J. Trace Elem. Med. Biol. 2023, 76, 127124. [Google Scholar] [CrossRef]
- Lima, L.G.; Santos, A.A.M.D.; Gueiber, T.D.; Gomes, R.Z.; Martins, C.M.; Chaikoski, A.C. Relation between Selenium and Female Fertility: A Systematic Review. Rev. Bras. Ginecol. Obstet. 2022, 44, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.D.; Broughton Pipkin, F.; Redman, C.W.G.; Poston, L. Selenium in Reproductive Health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Camp, O.G.; Bembenek, J.N.; Goud, P.T.; Awonuga, A.O.; Abu-Soud, H.M. The Implications of Insufficient Zinc on the Generation of Oxidative Stress Leading to Decreased Oocyte Quality. Reprod. Sci. 2023, 30, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Brion, L.P.; Heyne, R.; Lair, C.S. Role of Zinc in Neonatal Growth and Brain Growth: Review and Scoping Review. Pediatr. Res. 2021, 89, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.-M.; Lin, X.-N.; Song, T.; Liu, L.; Zhang, S.-Y. Calcium-Binding Protein S100P Is Highly Expressed during the Implantation Window in Human Endometrium. Fertil. Steril. 2010, 94, 1510–1518. [Google Scholar] [CrossRef]
- Zhang, R.-J.; Zou, L.-B.; Zhang, D.; Tan, Y.-J.; Wang, T.-T.; Liu, A.-X.; Qu, F.; Meng, Y.; Ding, G.-L.; Lu, Y.-C.; et al. Functional Expression of Large-Conductance Calcium-Activated Potassium Channels in Human Endometrium: A Novel Mechanism Involved in Endometrial Receptivity and Embryo Implantation. J. Clin. Endocrinol. Metab. 2012, 97, 543–553. [Google Scholar] [CrossRef]
- Yılmaz, B.K.; Evliyaoğlu, Ö.; Yorgancı, A.; Özyer, Ş.; Üstün, Y.E. Serum Concentrations of Heavy Metals in Women with Endometrial Polyps. J. Obstet. Gynaecol. 2020, 40, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Onuma, T.; Mizutani, T.; Fujita, Y.; Ohgami, N.; Ohnuma, S.; Kato, M.; Yoshida, Y. Zinc Deficiency Is Associated with the Development of Ovarian Endometrial Cysts. Am. J. Cancer Res. 2023, 13, 1049–1066. [Google Scholar] [PubMed]
- Defrère, S.; Lousse, J.C.; González-Ramos, R.; Colette, S.; Donnez, J.; Van Langendonckt, A. Potential Involvement of Iron in the Pathogenesis of Peritoneal Endometriosis. Mol. Hum. Reprod. 2008, 14, 377–385. [Google Scholar] [CrossRef]
- Yao, Y.M.; Osuchowski, M.F.; Pan, Z.K.; Wang, J.H. Immune Dysfunction: An Update of New Immune Cell Subsets and Cytokines in Sepsis; Frontiers Media SA: Lausanne, Switzerland, 2022; ISBN 978-2-88-974248-6. [Google Scholar]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Iron Intake and Risk of Ovulatory Infertility. Obstet. Gynecol. 2006, 108, 1145–1152. [Google Scholar] [CrossRef]
- Li, Y.Q.; Cao, X.X.; Bai, B.; Zhang, J.N.; Wang, M.Q.; Zhang, Y.H. Severe Iron Deficiency Is Associated with a Reduced Conception Rate in Female Rats. Gynecol. Obstet. Investig. 2014, 77, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Díaz, R.; Blanes-Zamora, R.; Paz-Montelongo, S.; Gómez-Rodríguez, J.; Fiestas, S.R.; González-Weller, D.; Gutiérrez, Á.J.; Rubio, C.; Hardisson, A.; Niebla-Canelo, D.; et al. The Influence of Follicular Fluid Metals on Assisted Reproduction Outcome. Biol. Trace Elem. Res. 2023, 201, 5069–5082. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.K.; Singer, H.A.; Rembold, C.M. Magnesium Relaxes Arterial Smooth Muscle by Decreasing Intracellular Ca2+ without Changing Intracellular Mg2+. J. Clin. Investig. 1992, 89, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.R.; Franklin, R.; Quast, D.C.; Fraga, N.; Loftin, C.A.; Yates, L.; Harrison, V. Relation of Endometriosis and Neuromuscular Disease of the Gastrointestinal Tract: New Insights. Fertil. Steril. 1998, 70, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, W.; Miao, S.; Dong, X.; Zou, X. Effects of Zinc on Cell Proliferation, Zinc Transport, and Calcium Deposition in Primary Endometrial Epithelial Cells of Laying Hens In Vitro. Biol. Trace Elem. Res. 2021, 199, 4251–4259. [Google Scholar] [CrossRef] [PubMed]
- Hoşgörler, F.; Kızıldağ, S.; Ateş, M.; Argon, A.; Koç, B.; Kandis, S.; Güvendi, G.; Ilgin, R.; Uysal, N. The Chronic Use of Magnesium Decreases VEGF Levels in the Uterine Tissue in Rats. Biol. Trace Elem. Res. 2020, 196, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Chavarro, J.E.; Malspeis, S.; Willett, W.C.; Missmer, S.A. Dairy-Food, Calcium, Magnesium, and Vitamin D Intake and Endometriosis: A Prospective Cohort Study. Am. J. Epidemiol. 2013, 177, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Yalçın Bahat, P.; Ayhan, I.; Üreyen Özdemir, E.; İnceboz, Ü.; Oral, E. Dietary Supplements for Treatment of Endometriosis: A Review. Acta Biomed. 2022, 93, e2022159. [Google Scholar]
- Bhuyan, A.K.; Sarma, D.; Saikia, U.K. Selenium and the Thyroid: A Close-Knit Connection. Indian J. Endocrinol. Metab. 2012, 16, S354–S355. [Google Scholar] [CrossRef]
- Mojadadi, A.; Au, A.; Salah, W.; Witting, P.; Ahmad, G. Role for Selenium in Metabolic Homeostasis and Human Reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef]
- Harding, K.B.; Peña-Rosas, J.P.; Webster, A.C.; Yap, C.M.; Payne, B.A.; Ota, E.; De-Regil, L.M. Iodine Supplementation for Women during the Preconception, Pregnancy and Postpartum Period. Cochrane Database Syst. Rev. 2017, 3, CD011761. [Google Scholar] [CrossRef]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid Function and Human Reproductive Health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef]
- Dosiou, C. Thyroid and Fertility: Recent Advances. Thyroid 2020, 30, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Medenica, S.; Nedeljkovic, O.; Radojevic, N.; Stojkovic, M.; Trbojevic, B.; Pajovic, B. Thyroid Dysfunction and Thyroid Autoimmunity in Euthyroid Women in Achieving Fertility. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 977–987. [Google Scholar] [PubMed]
- Ferri, N.; Ulisse, S.; Aghini-Lombardi, F.; Graziano, F.M.; Di Mattia, T.; Russo, F.P.; Arizzi, M.; Baldini, E.; Trimboli, P.; Attanasio, D.; et al. Iodine Supplementation Restores Fertility of Sheep Exposed to Iodine Deficiency. J. Endocrinol. Investig. 2003, 26, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Papalou, O.; Kandaraki, E.A. The Role of Androgen Excess on Insulin Sensitivity in Women. Front. Horm. Res. 2019, 53, 50–64. [Google Scholar]
- Xu, Y.; Qiao, J. Association of Insulin Resistance and Elevated Androgen Levels with Polycystic Ovarian Syndrome (PCOS): A Review of Literature. J. Healthc. Eng. 2022, 2022, 9240569. [Google Scholar] [CrossRef] [PubMed]
- Sliwowska, J.H.; Fergani, C.; Gawałek, M.; Skowronska, B.; Fichna, P.; Lehman, M.N. Insulin: Its Role in the Central Control of Reproduction. Physiol. Behav. 2014, 133, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Vatier, C.; Christin-Maitre, S.; Vigouroux, C. Role of Insulin Resistance on Fertility—Focus on Polycystic Ovary Syndrome. Ann. Endocrinol. 2022, 83, 199–202. [Google Scholar] [CrossRef]
- Patel, S. Polycystic Ovary Syndrome (PCOS), an Inflammatory, Systemic, Lifestyle Endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef]
- Foroozanfard, F.; Jamilian, M.; Jafari, Z.; Khassaf, A.; Hosseini, A.; Khorammian, H.; Asemi, Z. Effects of Zinc Supplementation on Markers of Insulin Resistance and Lipid Profiles in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Exp. Clin. Endocrinol. Diabetes 2015, 123, 215–220. [Google Scholar] [PubMed]
- Mazaheri Nia, L.; Iravani, M.; Abedi, P.; Cheraghian, B. Effect of Zinc on Testosterone Levels and Sexual Function of Postmenopausal Women: A Randomized Controlled Trial. J. Sex Marital Ther. 2021, 47, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Klug, A. Zinc Finger Peptides for the Regulation of Gene Expression. J. Mol. Biol. 1999, 293, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Favier, A.E. The Role of Zinc in Reproduction. Hormonal Mechanisms. Biol. Trace Elem. Res. 1992, 32, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Michos, C.; Kalfakakou, V.; Karkabounas, S.; Kiortsis, D.; Evangelou, A. Changes in Copper and Zinc Plasma Concentrations during the Normal Menstrual Cycle in Women. Gynecol. Endocrinol. 2010, 26, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Scholl, T.O.; Hediger, M.L.; Schall, J.I.; Fischer, R.L.; Khoo, C.S. Low Zinc Intake during Pregnancy: Its Association with Preterm and Very Preterm Delivery. Am. J. Epidemiol. 1993, 137, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Y.-F.; Hao, J.-H.; Chen, Y.-H.; Su, P.-Y.; Wang, Y.; Yu, Z.; Fu, L.; Xu, Y.-Y.; Zhang, C.; et al. Maternal Zinc Deficiency during Pregnancy Elevates the Risks of Fetal Growth Restriction: A Population-Based Birth Cohort Study. Sci. Rep. 2015, 5, 11262. [Google Scholar] [CrossRef] [PubMed]
- Neggers, Y.H.; Cutter, G.R.; Acton, R.T.; Alvarez, J.O.; Bonner, J.L.; Goldenberg, R.L.; Go, R.C.; Roseman, J.M. A Positive Association between Maternal Serum Zinc Concentration and Birth Weight. Am. J. Clin. Nutr. 1990, 51, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Wahlin-Jacobsen, S. Testosterone in Women--the Clinical Significance. Lancet Diabetes Endocrinol. 2015, 3, 980–992. [Google Scholar] [CrossRef]
- Rothman, M.S.; Carlson, N.E.; Xu, M.; Wang, C.; Swerdloff, R.; Lee, P.; Goh, V.H.H.; Ridgway, E.C.; Wierman, M.E. Reexamination of Testosterone, Dihydrotestosterone, Estradiol and Estrone Levels across the Menstrual Cycle and in Postmenopausal Women Measured by Liquid Chromatography–tandem Mass Spectrometry. Steroids 2011, 76, 177–182. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, C.; Gu, W.; Liu, R.; Chen, D. Associations between Serum Copper, Zinc, Selenium Level and Sex Hormones among 6-19 Years Old Children and Adolescents in NHANES 2013–2016. Front. Endocrinol. 2022, 13, 924338. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Chung, K.C. Zinc Finger Protein 131 Inhibits Estrogen Signaling by Suppressing Estrogen Receptor α Homo-Dimerization. Biochem. Biophys. Res. Commun. 2013, 430, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Arao, Y.; Korach, K.S. The Physiological Role of Estrogen Receptor Functional Domains. Essays Biochem. 2021, 65, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Anthony, K.; Diaz, F.J. Transition Metal Chelator Induces Progesterone Production in Mouse Cumulus-Oocyte Complexes and Corpora Lutea. Biol. Trace Elem. Res. 2017, 176, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Barile, G.; Sica, G.; Montemurro, A.; Iacobelli, S.; Corradini, M. Levels of Estrogen and Progesterone Receptor in Human Endometrium during the Menstrual Cycle. Eur. J. Obstet. Gynecol. Reprod. Biol. 1979, 9, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Duncan, F.E.; Que, E.L.; Zhang, N.; Feinberg, E.C.; O’Halloran, T.V.; Woodruff, T.K. The Zinc Spark Is an Inorganic Signature of Human Egg Activation. Sci. Rep. 2016, 6, 24737. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Diaz, F.J. Acute Dietary Zinc Deficiency before Conception Compromises Oocyte Epigenetic Programming and Disrupts Embryonic Development. Dev. Biol. 2013, 376, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Lisle, R.S.; Anthony, K.; Randall, M.A.; Diaz, F.J. Oocyte-Cumulus Cell Interactions Regulate Free Intracellular Zinc in Mouse Oocytes. Reproduction 2013, 145, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Que, E.L.; Bleher, R.; Duncan, F.E.; Kong, B.Y.; Gleber, S.C.; Vogt, S.; Chen, S.; Garwin, S.A.; Bayer, A.R.; Dravid, V.P.; et al. Quantitative Mapping of Zinc Fluxes in the Mammalian Egg Reveals the Origin of Fertilization-Induced Zinc Sparks. Nat. Chem. 2015, 7, 130–139. [Google Scholar] [CrossRef]
- Kim, A.M.; Vogt, S.; O’Halloran, T.V.; Woodruff, T.K. Zinc Availability Regulates Exit from Meiosis in Maturing Mammalian Oocytes. Nat. Chem. Biol. 2010, 6, 674–681. [Google Scholar] [CrossRef]
- Que, E.L.; Duncan, F.E.; Bayer, A.R.; Philips, S.J.; Roth, E.W.; Bleher, R.; Gleber, S.C.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc Sparks Induce Physiochemical Changes in the Egg Zona Pellucida That Prevent Polyspermy. Integr. Biol. 2017, 9, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, M.L.; Kong, B.Y.; Kim, A.M.; O’Halloran, T.V.; Woodruff, T.K. A Zinc-Dependent Mechanism Regulates Meiotic Progression in Mammalian Oocytes. Biol. Reprod. 2012, 86, 114. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Diaz, F.J. Zinc Depletion Causes Multiple Defects in Ovarian Function during the Periovulatory Period in Mice. Endocrinology 2012, 153, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.Y.; Bernhardt, M.L.; Kim, A.M.; O’Halloran, T.V.; Woodruff, T.K. Zinc Maintains Prophase I Arrest in Mouse Oocytes through Regulation of the MOS-MAPK Pathway. Biol. Reprod. 2012, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ebisch, I.M.W.; Thomas, C.M.G.; Peters, W.H.M.; Braat, D.D.M.; Steegers-Theunissen, R.P.M. The Importance of Folate, Zinc and Antioxidants in the Pathogenesis and Prevention of Subfertility. Hum. Reprod. Update 2007, 13, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Wactawski-Wende, J.; Michels, K.A.; Schliep, K.C.; Plowden, T.C.; Chaljub, E.N.; Mumford, S.L. Dietary Minerals, Reproductive Hormone Levels and Sporadic Anovulation: Associations in Healthy Women with Regular Menstrual Cycles. Br. J. Nutr. 2018, 120, 81–89. [Google Scholar] [CrossRef]
- Akinloye, O.; Abbiyesuku, F.M.; Oguntibeju, O.O.; Arowojolu, A.O.; Truter, E.J. The Impact of Blood and Seminal Plasma Zinc and Copper Concentrations on Spermogram and Hormonal Changes in Infertile Nigerian Men. Reprod. Biol. 2011, 11, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Karunanithy, R.; Edirisinghe, W.R.; Roy, A.C.; Wong, P.C.; Ratnam, S.S. Human Follicular Fluid Levels of Calcium, Copper and Zinc. Gynecol. Obstet. Investig. 1987, 23, 129–132. [Google Scholar] [CrossRef]
- Menezo, Y.; Khatchadourian, C.; Gharib, A.; Hamidi, J.; Greenland, T.; Sarda, N. Regulation of S-Adenosyl Methionine Synthesis in the Mouse Embryo. Life Sci. 1989, 44, 1601–1609. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, L.; Guo, Z.; Sun, L.; Wang, L.; Wang, C.; Zuo, Z.; Qiu, H. Association of Serum Heavy Metals and Trace Element Concentrations with Reproductive Hormone Levels and Polycystic Ovary Syndrome in a Chinese Population. Biol. Trace Elem. Res. 2015, 167, 1–10. [Google Scholar] [CrossRef]
- Özkaya, M.O.; Nazıroğlu, M.; Barak, C.; Berkkanoglu, M. Effects of Multivitamin/mineral Supplementation on Trace Element Levels in Serum and Follicular Fluid of Women Undergoing in Vitro Fertilization (IVF). Biol. Trace Elem. Res. 2011, 139, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kanafchian, M.; Mahjoub, S.; Esmaeilzadeh, S.; Rahsepar, M.; Mosapour, A. Status of Serum Selenium and Zinc in Patients with the Polycystic Ovary Syndrome with and without Insulin Resistance. Middle East Fertil. Soc. J. 2018, 23, 241–245. [Google Scholar] [CrossRef]
- Özer, A.; Bakacak, M.; Kıran, H.; Ercan, Ö.; Köstü, B.; Kanat-Pektaş, M.; Kılınç, M.; Aslan, F. Increased Oxidative Stress Is Associated with Insulin Resistance and Infertility in Polycystic Ovary Syndrome. Ginekol. Pol. 2016, 87, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Cruz, K.J.C.; de Oliveira, A.R.S.; Morais, J.B.S.; Severo, J.S.; Mendes, P.M.V.; de Sousa Melo, S.R.; de Sousa, G.S.; Marreiro, D. do N. Zinc and Insulin Resistance: Biochemical and Molecular Aspects. Biol. Trace Elem. Res. 2018, 186, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc Status Is Associated with Inflammation, Oxidative Stress, Lipid, and Glucose Metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Tańska, K.; Gietka-Czernel, M.; Glinicki, P.; Kozakowski, J. Thyroid Autoimmunity and Its Negative Impact on Female Fertility and Maternal Pregnancy Outcomes. Front. Endocrinol. 2022, 13, 1049665. [Google Scholar] [CrossRef] [PubMed]
- Stuss, M.; Michalska-Kasiczak, M.; Sewerynek, E. The Role of Selenium in Thyroid Gland Pathophysiology. Endokrynol. Pol. 2017, 68, 440–465. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. Selenium and the Thyroid. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Bucci, I.; Giuliani, C.; Di Dalmazi, G.; Formoso, G.; Napolitano, G. Thyroid Autoimmunity in Female Infertility and Assisted Reproductive Technology Outcome. Front. Endocrinol. 2022, 13, 768363. [Google Scholar] [CrossRef]
- Goldsmith, R.E.; Sturgis, S.H.; Lerman, J.; Stanbury, J.B. The Menstrual Pattern in Thyroid Disease. J. Clin. Endocrinol. Metab. 1952, 12, 846–855. [Google Scholar] [CrossRef]
- Koutras, D.A. Disturbances of Menstruation in Thyroid Disease. Ann. N. Y. Acad. Sci. 1997, 816, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Fedail, J.S.; Zheng, K.; Wei, Q.; Kong, L.; Shi, F. Roles of Thyroid Hormones in Follicular Development in the Ovary of Neonatal and Immature Rats. Endocrine 2014, 46, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Poppe, K.; Glinoer, D. Thyroid Autoimmunity and Hypothyroidism before and during Pregnancy. Hum. Reprod. Update 2003, 9, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Thyroid Hormones and Female Reproduction. Biol. Reprod. 2018, 99, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Mintziori, G.; Kita, M.; Duntas, L.; Goulis, D.G. Consequences of Hyperthyroidism in Male and Female Fertility: Pathophysiology and Current Management. J. Endocrinol. Investig. 2016, 39, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Vissenberg, R.; Manders, V.D.; Mastenbroek, S.; Fliers, E.; Afink, G.B.; Ris-Stalpers, C.; Goddijn, M.; Bisschop, P.H. Pathophysiological Aspects of Thyroid Hormone Disorders/thyroid Peroxidase Autoantibodies and Reproduction. Hum. Reprod. Update 2015, 21, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, R.; Beckmann, M.W.; Oppelt, P.G.; Hoffmann, I.; Lotz, L.; Kuwert, T.; Mueller, A. Thyroid Hormone Receptors and Reproduction. J. Reprod. Immunol. 2011, 90, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Zagrodzki, P.; Ratajczak, R.; Wietecha-Posłuszny, R. The Interaction between Selenium Status, Sex Hormones, and Thyroid Metabolism in Adolescent Girls in the Luteal Phase of Their Menstrual Cycle. Biol. Trace Elem. Res. 2007, 120, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zagrodzki, P.; Ratajczak, R. Selenium Status, Sex Hormones, and Thyroid Function in Young Women. J. Trace Elem. Med. Biol. 2008, 22, 296–304. [Google Scholar] [CrossRef]
- Guastamacchia, E.; Giagulli, V.A.; Licchelli, B.; Triggiani, V. Selenium and Iodine in Autoimmune Thyroiditis. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 288–292. [Google Scholar] [CrossRef]
- Krassas, G.E.; Pontikides, N.; Kaltsas, T.; Papadopoulou, P.; Paunkovic, J.; Paunkovic, N.; Duntas, L.H. Disturbances of Menstruation in Hypothyroidism. Clin. Endocrinol. 1999, 50, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L.; Buck Louis, G.M.; Kannan, K.; Weck, J.; Wan, Y.; Maisog, J.; Giannakou, A.; Wu, Q.; Sundaram, R. Delayed Conception in Women with Low-Urinary Iodine Concentrations: A Population-Based Prospective Cohort Study. Hum. Reprod. 2018, 33, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.M.; Johnson, N.P.; Sim, R.G.; O’Sullivan, S.; Peart, J.M.; Hofman, P.L. Iodine and Fertility: Do We Know Enough? Hum. Reprod. 2021, 36, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global Epidemiology of Hyperthyroidism and Hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.T.; Delange, F. Damaged Reproduction: The Most Important Consequence of Iodine Deficiency. J. Clin. Endocrinol. Metab. 2001, 86, 2360–2363. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, E.; Pearce, E.N. Iodine Status and Supplementation Before, during, and after Pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101430. [Google Scholar] [CrossRef]
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’Orazi, V.; Tartaglia, F.; Tripodi, D.; Lori, E.; Gagliardi, F.; Praticò, M.; et al. Iodine: Its Role in Thyroid Hormone Biosynthesis and Beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.V.; Bhandarkar, S.D.; Chadha, M.; Balaiah, D.; Shah, R. Menstrual Irregularities and Lactation Failure May Precede Thyroid Dysfunction or Goitre. J. Postgrad. Med. 1993, 39, 137–141. [Google Scholar]
- Rosner, W.; Hryb, D.J.; Khan, M.S.; Nakhla, A.M.; Romas, N.A. Sex Hormone-Binding Globulin: Anatomy and Physiology of a New Regulatory System. J. Steroid Biochem. Mol. Biol. 1991, 40, 813–820. [Google Scholar] [CrossRef]
- Zhu, J.-L.; Chen, Z.; Feng, W.-J.; Long, S.-L.; Mo, Z.-C. Sex Hormone-Binding Globulin and Polycystic Ovary Syndrome. Clin. Chim. Acta 2019, 499, 142–148. [Google Scholar] [CrossRef]
- Unuane, D.; Velkeniers, B. Impact of Thyroid Disease on Fertility and Assisted Conception. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101378. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.M.; Ko, C.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels with Inflammatory Processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef] [PubMed]
- Holesh, J.E.; Bass, A.N.; Lord, M. Physiology, Ovulation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kanasaki, H.; Oride, A.; Mijiddorj, T.; Kyo, S. Role of Thyrotropin-Releasing Hormone in Prolactin-Producing Cell Models. Neuropeptides 2015, 54, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.C.; Haahr, T.; Martínez-Moya, M.; Humaidan, P. Gonadotropin-Releasing Hormone Agonist for Ovulation Trigger—OHSS Prevention and Use of Modified Luteal Phase Support for Fresh Embryo Transfer. Upsala J. Med. Sci. 2020, 125, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Karsch, F.J.; Bowen, J.M.; Caraty, A.; Evans, N.P.; Moenter, S.M. Gonadotropin-Releasing Hormone Requirements for Ovulation. Biol. Reprod. 1997, 56, 303–309. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Azuma, K.; Irahara, M.; Yasui, T.; Aono, T. Mechanism of Anovulation in Hyperprolactinemic Amenorrhea Determined by Pulsatile Gonadotropin-Releasing Hormone Injection Combined with Human Chorionic Gonadotropin. Fertil. Steril. 1994, 62, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, U.B. Hyperprolactinemia and Infertility: New Insights. J. Clin. Investig. 2012, 122, 3467–3468. [Google Scholar] [CrossRef]
- Vogt, A.-C.S.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M.F. On Iron Metabolism and Its Regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, M.-M.; Wang, J.; Xie, J.-X. Effect of Estrogen on Iron Metabolism in Mammals. Sheng Li Xue Bao 2016, 68, 637–643. [Google Scholar]
- Hamad, M.; Bajbouj, K.; Taneera, J. The Case for an Estrogen-Iron Axis in Health and Disease. Exp. Clin. Endocrinol. Diabetes 2020, 128, 270–277. [Google Scholar] [CrossRef]
- Song, Y.-S.; Annalora, A.J.; Marcus, C.B.; Jefcoate, C.R.; Sorenson, C.M.; Sheibani, N. Cytochrome P450 1B1: A Key Regulator of Ocular Iron Homeostasis and Oxidative Stress. Cells 2022, 11, 2930. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, C.; Tsuchiya, K.; Maeda, K. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors and Iron Metabolism. Int. J. Mol. Sci. 2023, 24, 3037. [Google Scholar] [CrossRef] [PubMed]
- Vranka, J.A.; Sakai, L.Y.; Bächinger, H.P. Prolyl 3-Hydroxylase 1, Enzyme Characterization and Identification of a Novel Family of Enzymes. J. Biol. Chem. 2004, 279, 23615–23621. [Google Scholar] [CrossRef] [PubMed]
- Pihlajaniemi, T.; Myllylä, R.; Kivirikko, K.I. Prolyl 4-Hydroxylase and Its Role in Collagen Synthesis. J. Hepatol. 1991, 13 (Suppl. S3), S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-W.; Lai, Z.-Z.; Yang, H.-L.; Yang, S.-L.; Wang, C.-J.; Ao, D.; Ruan, L.-Y.; Shen, H.-H.; Zhou, W.-J.; Mei, J.; et al. Collagen at the Maternal-Fetal Interface in Human Pregnancy. Int. J. Biol. Sci. 2020, 16, 2220–2234. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F. Extracellular and Intracellular Regulation of Calcium Homeostasis. Sci. World J. 2001, 1, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Petersen, O.H.; Petersen, C.C.; Kasai, H. Calcium and Hormone Action. Annu. Rev. Physiol. 1994, 56, 297–319. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Calcium Control of Neurotransmitter Release. Cold Spring Harb. Perspect. Biol. 2012, 4, a011353. [Google Scholar] [CrossRef]
- Jasoni, C.L.; Romanò, N.; Constantin, S.; Lee, K.; Herbison, A.E. Calcium Dynamics in Gonadotropin-Releasing Hormone Neurons. Front. Neuroendocrinol. 2010, 31, 259–269. [Google Scholar] [CrossRef]
- Pitkin, R.M.; Reynolds, W.A.; Williams, G.A.; Hargis, G.K. Calcium-Regulating Hormones during the Menstrual Cycle. J. Clin. Endocrinol. Metab. 1978, 47, 626–632. [Google Scholar] [CrossRef]
- Kadoura, S.; Alhalabi, M.; Nattouf, A.H. Effect of Calcium and Vitamin D Supplements as an Adjuvant Therapy to Metformin on Menstrual Cycle Abnormalities, Hormonal Profile, and IGF-1 System in Polycystic Ovary Syndrome Patients: A Randomized, Placebo-Controlled Clinical Trial. Adv. Pharmacol. Sci. 2019, 2019, 9680390. [Google Scholar] [CrossRef]
- Williams, C.L.; Smith, S.M. Calcium Dependence of Spontaneous Neurotransmitter Release. J. Neurosci. Res. 2018, 96, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Bazybek, N.; Wei, Y.; Ma, G. Advances in Encapsulating Gonadotropin-Releasing Hormone Agonists for Controlled Release: A Review. J. Microencapsul. 2022, 39, 452–466. [Google Scholar] [CrossRef]
- Scheuer, R.; Philipp, S.E.; Becker, A.; Nalbach, L.; Ampofo, E.; Montenarh, M.; Götz, C. Protein Kinase CK2 Controls Ca2.1-Dependent Calcium Currents and Insulin Release in Pancreatic β-Cells. Int. J. Mol. Sci. 2020, 21, 4668. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, V.L.; MacDonald, P.E.; Klip, A. The Cell Biology of Systemic Insulin Function. J. Cell Biol. 2018, 217, 2273–2289. [Google Scholar] [CrossRef]
- Bettocchi, C.; Rinaldi, M.; Sebastiani, F. GnRH in the Treatment of Hypogonadotropic Hypogonadism. Curr. Pharm. Des. 2021, 27, 2754–2756. [Google Scholar] [CrossRef]
- Barabás, K.; Szabó-Meleg, E.; Ábrahám, I.M. Effect of Inflammation on Female Gonadotropin-Releasing Hormone (GnRH) Neurons: Mechanisms and Consequences. Int. J. Mol. Sci. 2020, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Hayashi, T.; Harada, N. Post-Translational Dual Regulation of Cytochrome P450 Aromatase at the Catalytic and Protein Levels by Phosphorylation/dephosphorylation. FEBS J. 2014, 281, 4830–4840. [Google Scholar] [CrossRef]
- Balthazart, J.; Baillien, M.; Charlier, T.D.; Cornil, C.A.; Ball, G.F. Multiple Mechanisms Control Brain Aromatase Activity at the Genomic and Non-Genomic Level. J. Steroid Biochem. Mol. Biol. 2003, 86, 367–379. [Google Scholar] [CrossRef]
- Nelson, L.R.; Bulun, S.E. Estrogen Production and Action. J. Am. Acad. Dermatol. 2001, 45, S116–S124. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, R.P.; Tullar, P.E.; Nipp, R.D.; Castracane, V.D. Serum Magnesium Concentrations and Metabolic Variables in Polycystic Ovary Syndrome. Acta Obstet. Gynecol. Scand. 2011, 90, 452–458. [Google Scholar] [CrossRef]
- Chakraborty, P.; Ghosh, S.; Goswami, S.K.; Kabir, S.N.; Chakravarty, B.; Jana, K. Altered Trace Mineral Milieu Might Play an Aetiological Role in the Pathogenesis of Polycystic Ovary Syndrome. Biol. Trace Elem. Res. 2013, 152, 9–15. [Google Scholar] [CrossRef]
- Kanafchian, M.; Esmaeilzadeh, S.; Mahjoub, S.; Rahsepar, M.; Ghasemi, M. Status of Serum Copper, Magnesium, and Total Antioxidant Capacity in Patients with Polycystic Ovary Syndrome. Biol. Trace Elem. Res. 2020, 193, 111–117. [Google Scholar] [CrossRef]
- Sharma, P.; Kapoor, H.S.; Kaur, B.; Kamra, P.; Khetarpal, P. Investigation of the Association of Serum Trace Elements Concentrations and Serum Biochemical Parameters with the Risk of Polycystic Ovary Syndrome: A Case-Control Study. Biol. Trace Elem. Res. 2023, 202, 73–86. [Google Scholar] [CrossRef]
- Hruby, A.; Meigs, J.B.; O’Donnell, C.J.; Jacques, P.F.; McKeown, N.M. Higher Magnesium Intake Reduces Risk of Impaired Glucose and Insulin Metabolism and Progression from Prediabetes to Diabetes in Middle-Aged Americans. Diabetes Care 2014, 37, 419–427. [Google Scholar] [CrossRef]
- Gommers, L.M.M.; Hoenderop, J.G.J.; Bindels, R.J.M.; de Baaij, J.H.F. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes 2016, 65, 3–13. [Google Scholar] [CrossRef]
- Kostov, K. Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef] [PubMed]
- Noori, N.; Nafar, M.; Poorrezagholi, F.; Ahmadpoor, P.; Samadian, F.; Firouzan, A.; Einollahi, B. Dietary Intakes of Fiber and Magnesium and Incidence of Metabolic Syndrome in First Year after Renal Transplantation. J. Ren. Nutr. 2010, 20, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.P.; Sharma, R.; Bansal, D.D. Implications of Magnesium Deficiency in Type 2 Diabetes: A Review. Biol. Trace Elem. Res. 2010, 134, 119–129. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Sahebkar, A.; Rodríguez-Morán, M.; Guerrero-Romero, F. A Systematic Review and Meta-Analysis of Randomized Controlled Trials on the Effects of Magnesium Supplementation on Insulin Sensitivity and Glucose Control. Pharmacol. Res. 2016, 111, 272–282. [Google Scholar] [CrossRef]
- Meier, R.K. Polycystic Ovary Syndrome. Nurs. Clin. N. Am. 2018, 53, 407–420. [Google Scholar] [CrossRef]
- Hamilton, K.P.; Zelig, R.; Parker, A.R.; Haggag, A. Insulin Resistance and Serum Magnesium Concentrations among Women with Polycystic Ovary Syndrome. Curr. Dev. Nutr. 2019, 3, nzz108. [Google Scholar] [CrossRef]
- Jamilian, M.; Maktabi, M.; Asemi, Z. A Trial on The Effects of Magnesium-Zinc-Calcium-Vitamin D Co-Supplementation on Glycemic Control and Markers of Cardio-Metabolic Risk in Women with Polycystic Ovary Syndrome. Arch. Iran. Med. 2017, 20, 640–645. [Google Scholar]
- Szczuko, M.; Skowronek, M.; Zapałowska-Chwyć, M.; Starczewski, A. Quantitative Assessment of Nutrition in Patients with Polycystic Ovary Syndrome (PCOS). Rocz. Panstw. Zakl. Hig. 2016, 67, 419–426. [Google Scholar]
- Luo, X.; Cai, W.-Y.; Ma, H.-L.; Cong, J.; Chang, H.; Gao, J.-S.; Shen, W.-J.; Wang, Y.; Yang, X.-M.; Wu, X.-K. Associations of Serum Magnesium With Insulin Resistance and Testosterone in Women With Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 683040. [Google Scholar] [CrossRef]
- Shahmoradi, S.; Chiti, H.; Tavakolizadeh, M.; Hatami, R.; Motamed, N.; Ghaemi, M. The Effect of Magnesium Supplementation on Insulin Resistance and Metabolic Profiles in Women with Polycystic Ovary Syndrome: A Randomized Clinical Trial. Biol. Trace Elem. Res. 2023, 202, 941–946. [Google Scholar] [CrossRef]
- Leong, I. Reproductive Endocrinology: Restoring Ovarian Function. Nat. Rev. Endocrinol. 2018, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. Ovarian Antral Folliculogenesis during the Human Menstrual Cycle: A Review. Hum. Reprod. Update 2012, 18, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of Oxidative Stress in Female Reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Homer, H.A. The Role of Oocyte Quality in Explaining “Unexplained” Infertility. Semin. Reprod. Med. 2020, 38, 21–28. [Google Scholar] [CrossRef]
- Chou, C.-H.; Chen, M.-J. The Effect of Steroid Hormones on Ovarian Follicle Development. Vitam. Horm. 2018, 107, 155–175. [Google Scholar]
- Piette, P. The History of Natural Progesterone, the Never-Ending Story. Climacteric 2018, 21, 308–314. [Google Scholar] [CrossRef]
- Kagawa, H.; Sakurai, Y.; Horiuchi, R.; Kazeto, Y.; Gen, K.; Imaizumi, H.; Masuda, Y. Mechanism of Oocyte Maturation and Ovulation and Its Application to Seed Production in the Japanese Eel. Fish Physiol. Biochem. 2013, 39, 13–17. [Google Scholar] [CrossRef]
- Maggi, R.; Cariboni, A.M.; Marelli, M.M.; Moretti, R.M.; Andrè, V.; Marzagalli, M.; Limonta, P. GnRH and GnRH Receptors in the Pathophysiology of the Human Female Reproductive System. Hum. Reprod. Update 2016, 22, 358–381. [Google Scholar] [CrossRef]
- Sánchez, F.; Smitz, J. Molecular Control of Oogenesis. Biochim. Biophys. Acta 2012, 1822, 1896–1912. [Google Scholar] [CrossRef]
- Krajnik, K.; Mietkiewska, K.; Skowronska, A.; Kordowitzki, P.; Skowronski, M.T. Oogenesis in Women: From Molecular Regulatory Pathways and Maternal Age to Stem Cells. Int. J. Mol. Sci. 2023, 24, 6837. [Google Scholar] [CrossRef]
- Tosti, E. Calcium Ion Currents Mediating Oocyte Maturation Events. Reprod. Biol. Endocrinol. 2006, 4, 26. [Google Scholar] [CrossRef]
- Homa, S.T.; Carroll, J.; Swann, K. The Role of Calcium in Mammalian Oocyte Maturation and Egg Activation. Hum. Reprod. 1993, 8, 1274–1281. [Google Scholar] [CrossRef]
- Boni, R.; Gualtieri, R.; Talevi, R.; Tosti, E. Calcium and Other Ion Dynamics during Gamete Maturation and Fertilization. Theriogenology 2007, 68 (Suppl. S1), S156–S164. [Google Scholar] [CrossRef]
- Silvestre, F.; Boni, R.; Fissore, R.A.; Tosti, E. Ca2+ Signaling during Maturation of Cumulus-Oocyte Complex in Mammals. Mol. Reprod. Dev. 2011, 78, 744–756. [Google Scholar] [CrossRef]
- Machaca, K. Ca2+ Signaling Differentiation during Oocyte Maturation. J. Cell. Physiol. 2007, 213, 331–340. [Google Scholar] [CrossRef]
- Ducibella, T.; Huneau, D.; Angelichio, E.; Xu, Z.; Schultz, R.M.; Kopf, G.S.; Fissore, R.; Madoux, S.; Ozil, J.-P. Egg-to-Embryo Transition Is Driven by Differential Responses to Ca2+ Oscillation Number. Dev. Biol. 2002, 250, 280–291. [Google Scholar] [CrossRef]
- Martín-Romero, F.J.; López-Guerrero, A.M.; Alvarez, I.S.; Pozo-Guisado, E. Role of Store-Operated Calcium Entry during Meiotic Progression and Fertilization of Mammalian Oocytes. Int. Rev. Cell Mol. Biol. 2012, 295, 291–328. [Google Scholar]
- Madgwick, S.; Levasseur, M.; Jones, K.T. Calmodulin-Dependent Protein Kinase II, and Not Protein Kinase C, Is Sufficient for Triggering Cell-Cycle Resumption in Mammalian Eggs. J. Cell Sci. 2005, 118, 3849–3859. [Google Scholar] [CrossRef]
- Lefèvre, B.; Pesty, A.; Courtot, A.-M.; Martins, C.V.C.; Broca, O.; Denys, A.; Arnault, E.; Poirot, C.; Avazeri, N. The Phosphoinositide-Phospholipase C (PI-PLC) Pathway in the Mouse Oocyte. Crit. Rev. Eukaryot. Gene Expr. 2007, 17, 259–269. [Google Scholar] [CrossRef]
- Webb, S.E.; Miller, A.L. Calcium Signalling during Zebrafish Embryonic Development. Bioessays 2000, 22, 113–123. [Google Scholar] [CrossRef]
- Islam, M.S. Calcium Signaling: From Basic to Bedside. Adv. Exp. Med. Biol. 2020, 1131, 1–6. [Google Scholar]
- Cao, X.; Chen, Y. Mitochondria and Calcium Signaling in Embryonic Development. Semin. Cell Dev. Biol. 2009, 20, 337–345. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, H.; Lv, J.; Dong, Y.; Zhao, M.; Sui, X.; Cui, R.; Liu, B.; Wu, K. Calcium Ionophore Improves Embryonic Development and Pregnancy Outcomes in Patients with Previous Developmental Problems in ICSI Cycles. BMC Pregnancy Childbirth 2022, 22, 894. [Google Scholar] [CrossRef] [PubMed]
- Takaya, J. Calcium-Deficiency during Pregnancy Affects Insulin Resistance in Offspring. Int. J. Mol. Sci. 2021, 22, 7008. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, P.; Tihtonen, K.; Isojärvi, J.; Ojala, R.; Ashorn, U.; Ashorn, P.; Tammela, O. Calcium Supplementation during Pregnancy and Long-Term Offspring Outcome: A Systematic Literature Review and Meta-Analysis. Ann. N. Y. Acad. Sci. 2022, 1510, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Appelman-Dijkstra, N.M.; Ertl, D.-A.; Zillikens, M.C.; Rjenmark, L.; Winter, E.M. Hypercalcemia during Pregnancy: Management and Outcomes for Mother and Child. Endocrine 2021, 71, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Ajong, A.B.; Kenfack, B.; Ali, I.M.; Yakum, M.N.; Ukaogo, P.O.; Mangala, F.N.; Aljerf, L.; Telefo, P.B. Calcium Supplementation in Pregnancy: An Analysis of Potential Determinants in an under-Resourced Setting. PLoS ONE 2023, 18, e0292303. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tang, Y.; Qin, H.; Meng, R.; Zhang, C.; Zhang, Y.; Yang, Y.; Qiao, P.; Liu, J.; Su, J. Zinc Supplementation Promotes Oocyte Maturation and Subsequent Embryonic Development in Sheep. Theriogenology 2023, 206, 161–169. [Google Scholar] [CrossRef]
- Garner, T.B.; Hester, J.M.; Carothers, A.; Diaz, F.J. Role of Zinc in Female Reproduction. Biol. Reprod. 2021, 104, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Petrucco, S.; Percudani, R. Structural Recognition of DNA by poly(ADP-Ribose)polymerase-like Zinc Finger Families. FEBS J. 2008, 275, 883–893. [Google Scholar] [CrossRef]
- Popović-Bijelić, A.; Kowol, C.R.; Lind, M.E.S.; Luo, J.; Himo, F.; Enyedy, E.A.; Arion, V.B.; Gräslund, A. Ribonucleotide Reductase Inhibition by Metal Complexes of Triapine (3-Aminopyridine-2-Carboxaldehyde Thiosemicarbazone): A Combined Experimental and Theoretical Study. J. Inorg. Biochem. 2011, 105, 1422–1431. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Kudo, H.; Suzuki, S.; Nemoto, N.; Sassa, S.; Sakamoto, S. Down Regulation by a Low-Zinc Diet in Gene Expression of Rat Prostatic Thymidylate Synthase and Thymidine Kinase. Nutr. Metab. 2008, 5, 12. [Google Scholar] [CrossRef]
- Wu, F.Y.; Wu, C.W. Zinc in DNA Replication and Transcription. Annu. Rev. Nutr. 1987, 7, 251–272. [Google Scholar] [CrossRef]
- MacDonald, R.S. The Role of Zinc in Growth and Cell Proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc Finger Proteins: New Insights into Structural and Functional Diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Ha, J.-H.; Prela, O.; Carpizo, D.R.; Loh, S.N. p53 and Zinc: A Malleable Relationship. Front. Mol. Biosci. 2022, 9, 895887. [Google Scholar] [CrossRef]
- Beyersmann, D.; Haase, H. Functions of Zinc in Signaling, Proliferation and Differentiation of Mammalian Cells. Biometals 2001, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Hester, J.; Hanna-Rose, W.; Diaz, F. Zinc Deficiency Reduces Fertility in C. Elegans Hermaphrodites and Disrupts Oogenesis and Meiotic Progression. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 191, 203–209. [Google Scholar] [CrossRef]
- Liao, X.; Wu, L.; Yin, D.; Tian, D.; Zhou, C.; Liu, J.; Li, S.; Zhou, J.; Nie, Y.; Liao, H.; et al. The Role of Zinc in Follicular Development. Mol. Biol. Rep. 2023, 50, 4527–4534. [Google Scholar] [CrossRef]
- Jeon, Y.; Yoon, J.D.; Cai, L.; Hwang, S.-U.; Kim, E.; Zheng, Z.; Lee, E.; Kim, D.Y.; Hyun, S.-H. Supplementation of Zinc on Oocyte in Vitro Maturation Improves Preimplatation Embryonic Development in Pigs. Theriogenology 2014, 82, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.Y.; Duncan, F.E.; Que, E.L.; Xu, Y.; Vogt, S.; O’Halloran, T.V.; Woodruff, T.K. The Inorganic Anatomy of the Mammalian Preimplantation Embryo and the Requirement of Zinc during the First Mitotic Divisions. Dev. Dyn. 2015, 244, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Hanna, L.A.; Clegg, M.S.; Momma, T.Y.; Daston, G.P.; Rogers, J.M.; Keen, C.L. Zinc Influences the in Vitro Development of Peri-Implantation Mouse Embryos. Birth Defects Res. A Clin. Mol. Teratol. 2003, 67, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Diaz, F.J. Zinc Deficiency During Oocyte Maturation Causes Defects in Preimplantation Embryonic Development. Biol. Reprod. 2012, 87, 199. [Google Scholar] [CrossRef]
- Taylor, K.M.; Muraina, I.A.; Brethour, D.; Schmitt-Ulms, G.; Nimmanon, T.; Ziliotto, S.; Kille, P.; Hogstrand, C. Zinc Transporter ZIP10 Forms a Heteromer with ZIP6 Which Regulates Embryonic Development and Cell Migration. Biochem. J. 2016, 473, 2531–2544. [Google Scholar] [CrossRef]
- Kim, A.M.; Bernhardt, M.L.; Kong, B.Y.; Ahn, R.W.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc Sparks Are Triggered by Fertilization and Facilitate Cell Cycle Resumption in Mammalian Eggs. ACS Chem. Biol. 2011, 6, 716–723. [Google Scholar] [CrossRef]
- Zhang, N.; Duncan, F.E.; Que, E.L.; O’Halloran, T.V.; Woodruff, T.K. The Fertilization-Induced Zinc Spark Is a Novel Biomarker of Mouse Embryo Quality and Early Development. Sci. Rep. 2016, 6, 22772. [Google Scholar] [CrossRef]
- Wooldridge, L.K.; Nardi, M.E.; Ealy, A.D. Zinc Supplementation during in Vitro Embryo Culture Increases Inner Cell Mass and Total Cell Numbers in Bovine blastocysts1. J. Anim. Sci. 2019, 97, 4946–4950. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Yoon, J.D.; Cai, L.; Hwang, S.-U.; Kim, E.; Zheng, Z.; Jeung, E.; Lee, E.; Hyun, S.-H. Zinc Deficiency during in Vitro Maturation of Porcine Oocytes Causes Meiotic Block and Developmental Failure. Mol. Med. Rep. 2015, 12, 5973–5982. [Google Scholar] [CrossRef] [PubMed]
- Janati, S.; Behmanesh, M.A.; Najafzadehvarzi, H.; Akhundzade, Z.; Poormoosavi, S.M. Follicular Fluid Zinc Level and Oocyte Maturity and Embryo Quality in Women with Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2021, 15, 197–201. [Google Scholar]
- Pang, W.; Leng, X.; Lu, H.; Yang, H.; Song, N.; Tan, L.; Jiang, Y.; Guo, C. Depletion of Intracellular Zinc Induces Apoptosis of Cultured Hippocampal Neurons through Suppression of ERK Signaling Pathway and Activation of Caspase-3. Neurosci. Lett. 2013, 552, 140–145. [Google Scholar] [CrossRef]
- Johnson-Wimbley, T.D.; Graham, D.Y. Diagnosis and Management of Iron Deficiency Anemia in the 21st Century. Therap. Adv. Gastroenterol. 2011, 4, 177–184. [Google Scholar] [CrossRef]
- Clark, A.R.; Stokes, Y.M. Follicle Structure Influences the Availability of Oxygen to the Oocyte in Antral Follicles. Comput. Math. Methods Med. 2011, 2011, 287186. [Google Scholar] [CrossRef]
- Liang, J.; Gao, Y.; Feng, Z.; Zhang, B.; Na, Z.; Li, D. Reactive Oxygen Species and Ovarian Diseases: Antioxidant Strategies. Redox Biol. 2023, 62, 102659. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Kyoya, T.; Nakamura, Y.; Sato, E.; Tomiyama, T.; Kyono, K. Oxygen Consumption Rate of Early Pre-Antral Follicles from Vitrified Human Ovarian Cortical Tissue. J. Reprod. Dev. 2014, 60, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Iron Deficiency in Pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K.; Krebs, N.F.; Cusick, S.E. The Benefits and Risks of Iron Supplementation in Pregnancy and Childhood. Annu. Rev. Nutr. 2019, 39, 121–146. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The Elemental Role of Iron in DNA Synthesis and Repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Dhorajia, V.V.; Kim, J.; Kim, Y. Mitochondrial Iron Metabolism and Neurodegenerative Diseases. Neurotoxicology 2022, 88, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Holzer, I.; Ott, J.; Beitl, K.; Mayrhofer, D.; Heinzl, F.; Ebenbauer, J.; Parry, J.P. Iron Status in Women with Infertility and Controls: A Case-Control Study. Front. Endocrinol. 2023, 14, 1173100. [Google Scholar] [CrossRef]
- Gonzalez-Martin, R.; Palomar, A.; Quiñonero, A.; Pellicer, N.; Fernandez-Saavedra, R.; Conde-Vilda, E.; Quejido, A.J.; Whitehead, C.; Scott, R.T., Jr.; Dominguez, F. The Impact of Essential Trace Elements on Ovarian Response and Reproductive Outcomes Following Single Euploid Embryo Transfer. Int. J. Mol. Sci. 2023, 24, 10968. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.N.; Chen, L.; Xu, T.M.; Zhang, K. Potential Clinical Implications of Iron Metabolism in Ovarian Endometriosis. J. Trace Elem. Med. Biol. 2022, 73, 127017. [Google Scholar] [CrossRef]
- Xu, G.; Chen, L.; Li, Q. Association of Iron Metabolism Markers, Socioeconomic and Lifestyle Factors with Endometriosis: A Cross-Sectional Study. J. Trace Elem. Med. Biol. 2023, 78, 127175. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and Pathophysiology of Endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of Endometriosis: The Genetic/epigenetic Theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef]
- Clower, L.; Fleshman, T.; Geldenhuys, W.J.; Santanam, N. Targeting Oxidative Stress Involved in Endometriosis and Its Pain. Biomolecules 2022, 12, 1055. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, X.; Lu, D.; Yin, M.; Shan, M.; Gao, Y. Erastin Induces Ferroptosis via Ferroportin-Mediated Iron Accumulation in Endometriosis. Hum. Reprod. 2021, 36, 951–964. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative Stress and Male Infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Paramio, M.-T. Impact of Oxidative Stress on Oocyte Competence for in Vitro Embryo Production Programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative Stress in Oocyte Aging and Female Reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The Effects of Oxidative Stress on Female Reproduction: A Review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Assaf, L.; Eid, A.A.; Nassif, J. Role of AMPK/mTOR, Mitochondria, and ROS in the Pathogenesis of Endometriosis. Life Sci. 2022, 306, 120805. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A Brief Insight into the Etiology, Genetics, and Immunology of Polycystic Ovarian Syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Siddiqui, S.A.; Uddin, M.G.; Ibrahim, M.; Uddin, S.M.N.; Adnan, M.T.; Rahaman, M.Z.; Kar, A.; Islam, M.S. Increased Oxidative Stress, Altered Trace Elements, and Macro-Minerals Are Associated with Female Obesity. Biol. Trace Elem. Res. 2020, 197, 384–393. [Google Scholar] [CrossRef]
- Samimi, M.; Pourhanifeh, M.H.; Mehdizadehkashi, A.; Eftekhar, T.; Asemi, Z. The Role of Inflammation, Oxidative Stress, Angiogenesis, and Apoptosis in the Pathophysiology of Endometriosis: Basic Science and New Insights Based on Gene Expression. J. Cell. Physiol. 2019, 234, 19384–19392. [Google Scholar] [CrossRef] [PubMed]
- Zejnullahu, V.A.; Zejnullahu, V.A.; Kosumi, E. The Role of Oxidative Stress in Patients with Recurrent Pregnancy Loss: A Review. Reprod. Health 2021, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef]
- Joo, E.H.; Kim, Y.R.; Kim, N.; Jung, J.E.; Han, S.H.; Cho, H.Y. Effect of Endogenic and Exogenic Oxidative Stress Triggers on Adverse Pregnancy Outcomes: Preeclampsia, Fetal Growth Restriction, Gestational Diabetes Mellitus and Preterm Birth. Int. J. Mol. Sci. 2021, 22, 10122. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron Homeostasis and Oxidative Stress: An Intimate Relationship. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and Selenoproteins: It’s Role in Regulation of Inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Powell, S.R. The Antioxidant Properties of Zinc. J. Nutr. 2000, 130, 1447S–1454S. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and Inflammation: Advances and Perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef]
- Khalid, H.; Hanif, M.; Hashmi, M.A.; Mahmood, T.; Ayub, K.; Monim-Ul-Mehboob, M. Copper Complexes of Bioactive Ligands with Superoxide Dismutase Activity. Mini Rev. Med. Chem. 2013, 13, 1944–1956. [Google Scholar] [CrossRef]
- Ferronato, G.A.; Alvarado-Rincón, J.A.; Maffi, A.S.; Barbosa, A.A.; Gasperin, B.G.; Schneider, A.; Mondadori, R.G.; Brauner, C.C.; Corrêa, M.N. Expression of Genes Associated with Fertility in the Uterus and Oviduct of Heifers Challenged with Lipopolysaccharide. Zygote 2022, 30, 584–587. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc Deficiency and Cellular Oxidative Stress: Prognostic Implications in Cardiovascular Diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Impact of the Discovery of Human Zinc Deficiency on Health. J. Trace Elem. Med. Biol. 2014, 28, 357–363. [Google Scholar] [CrossRef]
- Ota, E.; Mori, R.; Middleton, P.; Tobe-Gai, R.; Mahomed, K.; Miyazaki, C.; Bhutta, Z.A. Zinc Supplementation for Improving Pregnancy and Infant Outcome. Cochrane Database Syst. Rev. 2015, 2015, CD000230. [Google Scholar] [CrossRef] [PubMed]
- Gohari, H.; Khajavian, N.; Mahmoudian, A.; Bilandi, R.R. Copper and Zinc Deficiency to the Risk of Preterm Labor in Pregnant Women: A Case-Control Study. BMC Pregnancy Childbirth 2023, 23, 366. [Google Scholar] [CrossRef] [PubMed]
- Ménézo, Y.; Pluntz, L.; Chouteau, J.; Gurgan, T.; Demirol, A.; Dalleac, A.; Benkhalifa, M. Zinc Concentrations in Serum and Follicular Fluid during Ovarian Stimulation and Expression of Zn2+ Transporters in Human Oocytes and Cumulus Cells. Reprod. Biomed. Online 2011, 22, 647–652. [Google Scholar] [CrossRef]
- Falchuk, K.H.; Montorzi, M. Zinc Physiology and Biochemistry in Oocytes and Embryos. Biometals 2001, 14, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Jose, N.; Elangovan, A.V.; Awachat, V.B.; Shet, D.; Ghosh, J.; David, C.G. Response of in Ovo Administration of Zinc on Egg Hatchability and Immune Response of Commercial Broiler Chicken. J. Anim. Physiol. Anim. Nutr. 2018, 102, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Muraina, I.A.; Maret, W.; Bury, N.R.; Hogstrand, C. Hatching Gland Development and Hatching in Zebrafish Embryos: A Role for Zinc and Its Transporters Zip10 and Znt1a. Biochem. Biophys. Res. Commun. 2020, 528, 698–705. [Google Scholar] [CrossRef]
- Tian, X.; Anthony, K.; Neuberger, T.; Diaz, F.J. Preconception Zinc Deficiency Disrupts Postimplantation Fetal and Placental Development in Mice. Biol. Reprod. 2014, 90, 83. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.K.; Wang, H.; Dey, S.K.; Palmiter, R.D. Mouse Zinc Transporter 1 Gene Provides an Essential Function during Early Embryonic Development. Genesis 2004, 40, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L.; Grieger, J.A.; Bianco-Miotto, T.; Roberts, C.T. Association between Maternal Zinc Status, Dietary Zinc Intake and Pregnancy Complications: A Systematic Review. Nutrients 2016, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Eide, D.J. The Oxidative Stress of Zinc Deficiency. Metallomics 2011, 3, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Messalli, E.M.; Schettino, M.T.; Mainini, G.; Ercolano, S.; Fuschillo, G.; Falcone, F.; Esposito, E.; Di Donna, M.C.; De Franciscis, P.; Torella, M. The Possible Role of Zinc in the Etiopathogenesis of Endometriosis. Clin. Exp. Obstet. Gynecol. 2014, 41, 541–546. [Google Scholar] [CrossRef]
- Mier-Cabrera, J.; Aburto-Soto, T.; Burrola-Méndez, S.; Jiménez-Zamudio, L.; Tolentino, M.C.; Casanueva, E.; Hernández-Guerrero, C. Women with Endometriosis Improved Their Peripheral Antioxidant Markers after the Application of a High Antioxidant Diet. Reprod. Biol. Endocrinol. 2009, 7, 54. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and Its Role in Immunity and Inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc Requirements and the Risks and Benefits of Zinc Supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Maret, W. Imbalance between pro-Oxidant and pro-Antioxidant Functions of Zinc in Disease. J. Alzheimers Dis. 2005, 8, 161–170; discussion 209–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid. Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, U.; Kamran, Z.; Raza, I.; Ahmad, S.; Babar, W.; Riaz, M.H.; Iqbal, Z. Role of Selenium in Male Reproduction—A Review. Anim. Reprod. Sci. 2014, 146, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Boitani, C.; Puglisi, R. Selenium, a Key Element in Spermatogenesis and Male Fertility. Adv. Exp. Med. Biol. 2008, 636, 65–73. [Google Scholar] [PubMed]
- Singh, A.K.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Markers of Oxidative Stress in Follicular Fluid of Women with Endometriosis and Tubal Infertility Undergoing IVF. Reprod. Toxicol. 2013, 42, 116–124. [Google Scholar] [CrossRef]
- Adeniran, S.O.; Zheng, P.; Feng, R.; Adegoke, E.O.; Huang, F.; Ma, M.; Wang, Z.; Ifarajimi, O.O.; Li, X.; Zhang, G. The Antioxidant Role of Selenium via GPx1 and GPx4 in LPS-Induced Oxidative Stress in Bovine Endometrial Cells. Biol. Trace Elem. Res. 2022, 200, 1140–1155. [Google Scholar] [CrossRef] [PubMed]
- Crites, B.R. The Effects of Form of Selenium on the Bovine Corpus Luteum, Uterine Endometrium, and Development of the Conceptus. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2021. [Google Scholar]
- Jankowski, C.S.R.; Rabinowitz, J.D. Selenium Modulates Cancer Cell Response to Pharmacologic Ascorbate. Cancer Res. 2022, 82, 3486–3498. [Google Scholar] [CrossRef]
- Selenium: An Essential Element for Glutathione Peroxidase Activity. Nutr. Rev. 1973, 31, 289–291.
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The Role of Oxidative Stress in Ovarian Aging: A Review. J. Ovarian Res. 2022, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Luderer, U. Oxidative Damage Increases and Antioxidant Gene Expression Decreases with Aging in the Mouse Ovary. Biol. Reprod. 2011, 84, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Timóteo-Ferreira, F.; Abreu, D.; Mendes, S.; Matos, L.; Rodrigues, A.R.; Almeida, H.; Silva, E. Redox Imbalance in Age-Related Ovarian Dysfunction and Perspectives for Its Prevention. Ageing Res. Rev. 2021, 68, 101345. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the Role of Glutathione on Oxidative Stress and Infertility. JBRA Assist. Reprod. 2018, 22, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Grazul-Bilska, A.T.; Caton, J.S.; Arndt, W.; Burchill, K.; Thorson, C.; Borowczyk, E.; Bilski, J.J.; Redmer, D.A.; Reynolds, L.P.; Vonnahme, K.A. Cellular Proliferation and Vascularization in Ovine Fetal Ovaries: Effects of Undernutrition and Selenium in Maternal Diet. Reproduction 2009, 137, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Tamanini, C. Selenium Stimulates Estradiol Production in Bovine Granulosa Cells: Possible Involvement of Nitric Oxide. Domest. Anim. Endocrinol. 2000, 18, 1–17. [Google Scholar] [CrossRef]
- Ceko, M.J.; Hummitzsch, K.; Hatzirodos, N.; Bonner, W.M.; Aitken, J.B.; Russell, D.L.; Lane, M.; Rodgers, R.J.; Harris, H.H. X-Ray Fluorescence Imaging and Other Analyses Identify Selenium and GPX1 as Important in Female Reproductive Function. Metallomics 2015, 7, 71–82. [Google Scholar] [CrossRef]
- Pieczyńska, J.; Grajeta, H. The Role of Selenium in Human Conception and Pregnancy. J. Trace Elem. Med. Biol. 2015, 29, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hernández Guerrero, C.A.; Bujalil Montenegro, L.; de la Jara Díaz, J.; Mier Cabrera, J.; Bouchán Valencia, P. Endometriosis and deficient intake of antioxidants molecules related to peripheral and peritoneal oxidative stress. Ginecol. Obstet. Mex. 2006, 74, 20–28. [Google Scholar]
- Morgia, G.; Cimino, S.; Favilla, V.; Russo, G.I.; Squadrito, F.; Mucciardi, G.; Masieri, L.; Minutoli, L.; Grosso, G.; Castelli, T. Effects of Serenoa Repens, Selenium and Lycopene (Profluss®) on Chronic Inflammation Associated with Benign Prostatic Hyperplasia: Results of “FLOG” (Flogosis and Profluss in Prostatic and Genital Disease), a Multicentre Italian Study. Int. Braz. J. Urol. 2013, 39, 214–221. [Google Scholar] [CrossRef]
- Harris, E.D. Copper as a Cofactor and Regulator of Copper,zinc Superoxide Dismutase. J. Nutr. 1992, 122, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Solier, S.; Müller, S.; Cañeque, T.; Versini, A.; Mansart, A.; Sindikubwabo, F.; Baron, L.; Emam, L.; Gestraud, P.; Pantoș, G.D.; et al. A Druggable Copper-Signalling Pathway That Drives Inflammation. Nature 2023, 617, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kang, Y.; Chang, Q.; Zhang, B.; Liu, X.; Zeng, L.; Yan, H.; Dang, S. Maternal Zinc, Copper, and Selenium Intakes during Pregnancy and Congenital Heart Defects. Nutrients 2022, 14, 1055. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xiao, P.; Qiu, B.; Yu, H.-F.; Teng, C.-B. Copper Chaperone Antioxidant 1: Multiple Roles and a Potential Therapeutic Target. J. Mol. Med. 2023, 101, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Michaluk, A.; Kochman, K. Involvement of Copper in Female Reproduction. Reprod. Biol. 2007, 7, 193–205. [Google Scholar] [PubMed]
- Gao, G.; Yi, J.; Zhang, M.; Xiong, J.; Geng, L.; Mu, C.; Yang, L. Effects of Iron and Copper in Culture Medium on Bovine Oocyte Maturation, Preimplantation Embryo Development, and Apoptosis of Blastocysts In Vitro. J. Reprod. Dev. 2007, 53, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, J.; Yoon, J.D.; Hwang, S.-U.; Cai, L.; Kim, M.; Kim, G.; Oh, D.; Kim, E.; Hyun, S.-H. The Effect of Copper Supplementation on in Vitro Maturation of Porcine Cumulus-Oocyte Complexes and Subsequent Developmental Competence after Parthenogenetic Activation. Theriogenology 2021, 164, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Glubb, D.M.; O’Mara, T.A. Dietary Factors and Endometrial Cancer Risk: A Mendelian Randomization Study. Nutrients 2023, 15, 603. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Gargano, M.; Cao, J.; Bronson, R.T.; Heimler, I.; Hutz, R.J. Reduced Fertility in Female Mice Lacking Copper-Zinc Superoxide Dismutase. J. Biol. Chem. 1998, 273, 7765–7769. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Nath, S.; Massanyi, P.; Stawarz, R.; Kacaniova, M.; Kolesarova, A. Copper-Induced Changes in Reproductive Functions: In Vivo and in Vitro Effects. Physiol. Res. 2016, 65, 11–22. [Google Scholar] [CrossRef]
- Galaris, D.; Pantopoulos, K. Oxidative Stress and Iron Homeostasis: Mechanistic and Health Aspects. Crit. Rev. Clin. Lab. Sci. 2008, 45, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T. Iron-Induced Oxidative Stress in Human Diseases. Cells 2022, 11, 2152. [Google Scholar] [CrossRef] [PubMed]
- Golfeyz, S.; Lewis, S.; Weisberg, I.S. Hemochromatosis: Pathophysiology, Evaluation, and Management of Hepatic Iron Overload with a Focus on MRI. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Guo, Z.; Hou, L.; Xu, J.; Du, T.; Xu, T.; Guo, F. Iron Homeostasis in Arthropathies: From Pathogenesis to Therapeutic Potential. Ageing Res. Rev. 2021, 72, 101481. [Google Scholar] [CrossRef] [PubMed]
- Gattermann, N.; Muckenthaler, M.U.; Kulozik, A.E.; Metzgeroth, G.; Hastka, J. The Evaluation of Iron Deficiency and Iron Overload. Dtsch. Arztebl. Int. 2021, 118, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Camaioni, A.; Ucci, M.A.; Campagnolo, L.; De Felici, M.; Klinger, F.G. Italian Society of Embryology, Reproduction and Research (SIERR) The Process of Ovarian Aging: It Is Not Just about Oocytes and Granulosa Cells. J. Assist. Reprod. Genet. 2022, 39, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.L.; Shukla, P.; Pagidas, K.; Ahmed, N.S.; Karri, S.; Gunn, D.D.; Hurd, W.W.; Singh, K.K. Mitochondria in Ovarian Aging and Reproductive Longevity. Ageing Res. Rev. 2020, 63, 101168. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian Aging: Mechanisms and Clinical Consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Nan, B.; Kong, S.; Harlow, S. Changes in Iron Measures over Menopause and Associations with Insulin Resistance. J. Women’s Health 2012, 21, 872–877. [Google Scholar] [CrossRef]
- Liu, M.; Wu, K.; Wu, Y. The Emerging Role of Ferroptosis in Female Reproductive Disorders. Biomed. Pharmacother. 2023, 166, 115415. [Google Scholar] [CrossRef]
- Li, X.; Duan, X.; Tan, D.; Zhang, B.; Xu, A.; Qiu, N.; Chen, Z. Iron Deficiency and Overload in Men and Woman of Reproductive Age, and Pregnant Women. Reprod. Toxicol. 2023, 118, 108381. [Google Scholar] [CrossRef] [PubMed]
- Fanni, D.; Gerosa, C.; Nurchi, V.M.; Manchia, M.; Saba, L.; Coghe, F.; Crisponi, G.; Gibo, Y.; Van Eyken, P.; Fanos, V.; et al. The Role of Magnesium in Pregnancy and in Fetal Programming of Adult Diseases. Biol. Trace Elem. Res. 2021, 199, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Role of Magnesium in Genomic Stability. Mutat. Res. 2001, 475, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Spasov, A.A. Magnesium Deficiency and Oxidative Stress: An Update. Biomedicine 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, R.; Alizadeh, M.; Asghari Jafarabadi, M.; Heidari, L.; Nikbakht, R.; Babaahmadi Rezaei, H.; Karandish, M. Effects of Melatonin And/or Magnesium Supplementation on Biomarkers of Inflammation and Oxidative Stress in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2022, 200, 1010–1019. [Google Scholar] [CrossRef]
- Hsu, J.M.; Rubenstein, B.; Paleker, A.G. Role of Magnesium in Glutathione Metabolism of Rat Erythrocytes. J. Nutr. 1982, 112, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Dickens, B.F.; Weglicki, W.B.; Li, Y.S.; Mak, I.T. Magnesium Deficiency in Vitro Enhances Free Radical-Induced Intracellular Oxidation and Cytotoxicity in Endothelial Cells. FEBS Lett. 1992, 311, 187–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Davies, L.R.; Martin, S.M.; Bawaney, I.M.; Buettner, G.R.; Kerber, R.E. Magnesium Reduces Free Radical Concentration and Preserves Left Ventricular Function after Direct Current Shocks. Resuscitation 2003, 56, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.A.; DeJong, S.C.; Martin, S.M.; Smith, R.S.; Buettner, G.R.; Kerber, R.E. Magnesium Reduces Free Radicals in an in Vivo Coronary Occlusion-Reperfusion Model. J. Am. Coll. Cardiol. 1998, 32, 536–539. [Google Scholar] [CrossRef]
- Morais, J.B.S.; Severo, J.S.; Santos, L.R.D.; de Sousa Melo, S.R.; de Oliveira Santos, R.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. Role of Magnesium in Oxidative Stress in Individuals with Obesity. Biol. Trace Elem. Res. 2017, 176, 20–26. [Google Scholar] [CrossRef]
- Zarean, E.; Tarjan, A. Effect of Magnesium Supplement on Pregnancy Outcomes: A Randomized Control Trial. Adv. Biomed. Res. 2017, 6, 109. [Google Scholar]
- Grossi, E.; Castiglioni, S.; Moscheni, C.; Antonazzo, P.; Cetin, I.; Savasi, V.M. Serum Magnesium and Calcium Levels in Infertile Women during a Cycle of Reproductive Assistance. Magnes. Res. 2017, 30, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Crosby, D.D.; Bain, E.; Crowther, C.A. Magnesium Supplementation in Pregnancy. Cochrane Database Syst. Rev. 2014, 2014, CD000937. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Feng, F.; Ma, X.; Zhang, R.; Li, L.; Yue, F.; Lv, M.; Liu, L. Progress of Oxidative Stress in Endometrium Decidualization. J. Obstet. Gynaecol. 2022, 42, 3429–3434. [Google Scholar] [CrossRef]
- Li, W.; Liu, C.; Yang, Q.; Zhou, Y.; Liu, M.; Shan, H. Oxidative Stress and Antioxidant Imbalance in Ovulation Disorder in Patients with Polycystic Ovary Syndrome. Front. Nutr. 2022, 9, 1018674. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, Y.; Zhu, T.; Lin, X.; Jing, X.; Zhang, J. Oral Magnesium Supplementation for the Prevention of Preeclampsia: A Meta-Analysis or Randomized Controlled Trials. Biol. Trace Elem. Res. 2022, 200, 3572–3581. [Google Scholar] [CrossRef]
- Pereira, A.C.; Martel, F. Oxidative Stress in Pregnancy and Fertility Pathologies. Cell Biol. Toxicol. 2014, 30, 301–312. [Google Scholar] [CrossRef]
- Studer, J.M.; Schweer, W.P.; Gabler, N.K.; Ross, J.W. Functions of Manganese in Reproduction. Anim. Reprod. Sci. 2022, 238, 106924. [Google Scholar] [CrossRef]
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese Metabolism in Humans. Front. Biosci. 2018, 23, 1655–1679. [Google Scholar] [CrossRef]
- Golara, A.; Kozłowski, M.; Guzik, P.; Kwiatkowski, S.; Cymbaluk-Płoska, A. The Role of Selenium and Manganese in the Formation, Diagnosis and Treatment of Cervical, Endometrial and Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 10887. [Google Scholar] [CrossRef]
- Tholin, K.; Palm, R.; Hallmans, G.; Sandström, B. Manganese Status during Pregnancy. Ann. N. Y. Acad. Sci. 1993, 678, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hivert, M.-F.; Rifas-Shiman, S.L.; Rahman, M.L.; Oken, E.; Cardenas, A.; Mueller, N.T. Prospective Association Between Manganese in Early Pregnancy and the Risk of Preeclampsia. Epidemiology 2020, 31, 677–680. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M. Oxygen radicals-superoxide dismutase system and reproduction medicine. Nihon Sanka Fujinka Gakkai Zasshi 1993, 45, 842–848. [Google Scholar] [PubMed]
- Sugino, N.; Takiguchi, S.; Kashida, S.; Karube, A.; Nakamura, Y.; Kato, H. Superoxide Dismutase Expression in the Human Corpus Luteum during the Menstrual Cycle and in Early Pregnancy. Mol. Hum. Reprod. 2000, 6, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Joo, B.S.; Na, Y.J.; Yoon, M.S.; Choi, O.H.; Kim, W.W. Relationships between Concentrations of Tumor Necrosis Factor-Alpha and Nitric Oxide in Follicular Fluid and Oocyte Quality. J. Assist. Reprod. Genet. 2000, 17, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Holden, E.C.; Dodge, L.E.; Sneeringer, R.; Moragianni, V.A.; Penzias, A.S.; Hacker, M.R. Thicker Endometrial Linings Are Associated with Better IVF Outcomes: A Cohort of 6331 Women. Hum. Fertil. 2018, 21, 288–293. [Google Scholar] [CrossRef]
- Blanco-Breindel, M.F.; Singh, M.; Kahn, J. Endometrial Receptivity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- Haas, J.; Casper, R.F. Observations on Clinical Assessment of Endometrial Receptivity. Fertil. Steril. 2022, 118, 828–831. [Google Scholar] [CrossRef]
- Bavan, B.; Gardner, R.M.; Zhang, W.Y.; Aghajanova, L. The Effect of Human Growth Hormone on Endometrial Growth in Controlled Ovarian Hyperstimulation Cycles. J. Pers. Med. 2022, 12, 1991. [Google Scholar] [CrossRef]
- Navot, D.; Scott, R.T.; Droesch, K.; Veeck, L.L.; Liu, H.C.; Rosenwaks, Z. The Window of Embryo Transfer and the Efficiency of Human Conception in Vitro. Fertil. Steril. 1991, 55, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Muter, J.; Lynch, V.J.; McCoy, R.C.; Brosens, J.J. Human Embryo Implantation. Development 2023, 150, dev201507. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, E.R.; Schust, D.J.; Fisher, S.J. Implantation and the Survival of Early Pregnancy. N. Engl. J. Med. 2001, 345, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Shafei, R.A.; Syrkasheva, A.G.; Romanov, A.Y.; Makarova, N.P.; Dolgushina, N.V.; Semenova, M.L. Blastocyst Hatching in Humans. Ontogenez 2017, 48, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Gauster, M.; Moser, G.; Wernitznig, S.; Kupper, N.; Huppertz, B. Early Human Trophoblast Development: From Morphology to Function. Cell. Mol. Life Sci. 2022, 79, 345. [Google Scholar] [CrossRef] [PubMed]
- Brosens, J.J.; Salker, M.S.; Teklenburg, G.; Nautiyal, J.; Salter, S.; Lucas, E.S.; Steel, J.H.; Christian, M.; Chan, Y.-W.; Boomsma, C.M.; et al. Uterine Selection of Human Embryos at Implantation. Sci. Rep. 2014, 4, 3894. [Google Scholar] [CrossRef] [PubMed]
- Scholl, T.O. Iron Status during Pregnancy: Setting the Stage for Mother and Infant. Am. J. Clin. Nutr. 2005, 81, 1218S–1222S. [Google Scholar] [CrossRef] [PubMed]
- Zachara, B.A.; Dobrzyński, W.; Trafikowska, U.; Szymański, W. Blood Selenium and Glutathione Peroxidases in Miscarriage. BJOG 2001, 108, 244–247. [Google Scholar] [PubMed]
- Pandur, E.; Pap, R.; Jánosa, G.; Horváth, A.; Sipos, K. Fractalkine Improves the Expression of Endometrium Receptivity-Related Genes and Proteins at Desferrioxamine-Induced Iron Deficiency in HEC-1A Cells. Int. J. Mol. Sci. 2023, 24, 7924. [Google Scholar] [CrossRef]
- Pandur, E.; Pap, R.; Montskó, G.; Jánosa, G.; Sipos, K.; Kovács, G.L. Fractalkine Enhances Endometrial Receptivity and Activates Iron Transport towards Trophoblast Cells in an in Vitro Co-Culture System of HEC-1A and JEG-3 Cells. Exp. Cell Res. 2021, 403, 112583. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc: An Overview. Nutrition 1995, 11, 93–99. [Google Scholar] [PubMed]
- Lu, X.; Zhang, Q.; Xu, L.; Lin, X.; Fu, J.; Wang, X.; Liu, Y.; Lin, Y.; Li, B.; Wang, R.; et al. Zinc Is Essential for the Transcription Function of the PGC-1α/Nrf2 Signaling Pathway in Human Primary Endometrial Stromal Cells. Am. J. Physiol.-Cell Physiol. 2020, 318, C640–C648. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.A.; Grzeskowiak, L.E.; Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Jankovic-Karasoulos, T.; Perkins, A.V.; Norman, R.J.; Dekker, G.A.; Roberts, C.T. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients 2019, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.-L.; Yeh, C.-C.; Yeh, C.-Y.; Chen, R.-Y.; Fu, C.-L.; Chen, C.-H.; Tzeng, C.-R. Decreased Zinc and Increased Lead Blood Levels Are Associated with Endometriosis in Asian Women. Reprod. Toxicol. 2017, 74, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jang, H.; Yoo, I.; Han, J.; Jung, W.; Ka, H. Unique Epithelial Expression of S100A Calcium Binding Protein A7A in the Endometrium at Conceptus Implantation in Pigs. Asian-Australas. J. Anim. Sci. 2019, 32, 1355–1362. [Google Scholar] [CrossRef]
- Choi, Y.; Jang, H.; Seo, H.; Yoo, I.; Han, J.; Kim, M.; Lee, S.; Ka, H. Changes in Calcium Levels in the Endometrium throughout Pregnancy and the Role of Calcium on Endometrial Gene Expression at the Time of Conceptus Implantation in Pigs. Mol. Reprod. Dev. 2019, 86, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Han, N.; Yang, H.; Wang, L.; Lin, X.; Diao, S.; Du, J.; Dong, R.; Wang, S.; Fan, Z. Homeobox C10 Inhibits the Osteogenic Differentiation Potential of Mesenchymal Stem Cells. Connect. Tissue Res. 2018, 59, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D. The Leukemia Inhibitory Factor (LIF). Stem Cells 1996, 9, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Huang, W.; Yuan, L.; Chen, S.; Liao, S.; Fu, X.; Liu, B.; Yang, Y. HOXA10 Improves Endometrial Receptivity by Upregulating E-Cadherin. Biol. Reprod. 2022, 106, 992–999. [Google Scholar] [CrossRef]
- Taylor, H.S.; Vanden Heuvel, G.B.; Igarashi, P. A Conserved Hox Axis in the Mouse and Human Female Reproductive System: Late Establishment and Persistent Adult Expression of the Hoxa Cluster Genes. Biol. Reprod. 1997, 57, 1338–1345. [Google Scholar] [CrossRef]
- Du, H.; Taylor, H.S. The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harb. Perspect. Med. 2015, 6, a023002. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, X.; Saravelos, S.H.; Liu, Y.; Huang, J.; Zhang, J.; Li, T.C. HOXA-10 and E-Cadherin Expression in the Endometrium of Women with Recurrent Implantation Failure and Recurrent Miscarriage. Fertil. Steril. 2017, 107, 136–143.e2. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.A.; Nafady, A.; Taha, S.A.M.; El-Din, A.M.G.; Ali, A.E.-N.A.E.-G. Leukemia Inhibitory Factor a Marker of Implantation Success in Unexplained Infertility: A Randomized Controlled Trial. Clin. Lab. 2022, 68, 2454. [Google Scholar] [CrossRef]
- Choi, K.C.; An, B.S.; Yang, H.; Jeung, E.B. Regulation and Molecular Mechanisms of Calcium Transport Genes: Do They Play a Role in Calcium Transport in the Uterine Endometrium? J. Physiol. Pharmacol. 2011, 62, 499–504. [Google Scholar]
- Nakamura, H.; Kimura, T.; Ogita, K.; Nakamura, T.; Takemura, M.; Shimoya, K.; Koyama, S.; Tsujie, T.; Koyama, M.; Murata, Y. NF-kappaB Activation at Implantation Window of the Mouse Uterus. Am. J. Reprod. Immunol. 2004, 51, 16–21. [Google Scholar] [CrossRef]
- Omer, F. Department of Reproduction and obstetrics, Faculty of Veterinary Medicine, University of Khartoum, Shambat, Sudan Intrauterine Infusion of Lugol’s Iodine Improves the Reproductive Traits of Postpartum Infected Dairy Cows. IOSR J. Agric. Vet. Sci. 2013, 5, 89–94. [Google Scholar] [CrossRef]
- Yoshida, R.; Kitahara, G.; Osawa, T. Intrauterine Infusion of Povidone-Iodine: Its Effect on the Endometrium and Subsequent Fertility in Postpartum Dairy Cows. J. Vet. Med. Sci. 2020, 82, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Mido, S.; Murata, N.; Rawy, M.S.; Kitahara, G.; Osawa, T. Effects of Intrauterine Infusion of Povidone-Iodine on Endometrial Cytology and Bacteriology in Dairy Cows with Clinical Endometritis. J. Vet. Med. Sci. 2016, 78, 551–556. [Google Scholar] [CrossRef]
- Johnson, N.P.; Bhattu, S.; Wagner, A.; Blake, D.A.; Chamley, L.W. Lipiodol Alters Murine Uterine Dendritic Cell Populations: A Potential Mechanism for the Fertility-Enhancing Effect of Lipiodol. Fertil. Steril. 2005, 83, 1814–1821. [Google Scholar] [CrossRef]
- Johnson, N.P.; Kwok, R.; Stewart, A.W.; Saththianathan, M.; Hadden, W.E.; Chamley, L.W. Lipiodol Fertility Enhancement: Two-Year Follow-up of a Randomized Trial Suggests a Transient Benefit in Endometriosis, but a Sustained Benefit in Unexplained Infertility. Hum. Reprod. 2007, 22, 2857–2862. [Google Scholar] [CrossRef]
- van Dyk, E.; Lange, A.L. The detrimental effect of iodine as an intra-uterine instillation in mares. J. S. Afr. Vet. Assoc. 1986, 57, 205–210. [Google Scholar] [PubMed]
- Wang, S.; Bu, Y.; Shao, Q.; Cai, Y.; Sun, D.; Fan, L. A Cohort Study on the Effects of Maternal High Serum Iodine Status During Pregnancy on Infants in Terms of Iodine Status and Intellectual, Motor, and Physical Development. Biol. Trace Elem. Res. 2023, 202, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Lazarus, J.H.; Moreno-Reyes, R.; Zimmermann, M.B. Consequences of Iodine Deficiency and Excess in Pregnant Women: An Overview of Current Knowns and Unknowns. Am. J. Clin. Nutr. 2016, 104 (Suppl. S3), 918S–923S. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B. Iodine Deficiency May Impair Fertility. JAMA 2018, 319, 760. [Google Scholar] [CrossRef] [PubMed]
- Seelig, M.S. Interrelationship of Magnesium and Estrogen in Cardiovascular and Bone Disorders, Eclampsia, Migraine and Premenstrual Syndrome. J. Am. Coll. Nutr. 1993, 12, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Yang, P.-K.; Chen, L.-C.; Cheong, M.-L.; Tsai, Y.-L.; Tsai, M.-S. Associations between Gene Expression of Magnesium Transporters and Glucose Metabolism in Pregnancy. J. Formos. Med. Assoc. 2022, 121, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh-Moghaddam, M.; Ghasemi-Tehrani, H.; Askari, G.; Jaripur, M.; Clark, C.C.T.; Rouhani, M.H. Effect of Magnesium Supplementation in Improving Hyperandrogenism, Hirsutism, and Sleep Quality in Women with Polycystic Ovary Syndrome: A Randomized, Placebo-Controlled Clinical Trial. Health Sci. Rep. 2023, 6, e1013. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Hypomagnesemia, Oxidative Stress, Inflammation, and Metabolic Syndrome. Diabetes Metab. Res. Rev. 2006, 22, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Aal-Hamad, A.H.; Al-Alawi, A.M.; Kashoub, M.S.; Falhammar, H. Hypermagnesemia in Clinical Practice. Medicina 2023, 59, 1190. [Google Scholar] [CrossRef]
- Simpson, W.G.; Vernon, M.E.; Jones, H.M.; Rush, M.E. The Role of Calcium in Gonadotropin-Releasing Hormone Induction of Follicle-Stimulating Hormone Release by the Pituitary Gonadotrope. Endocr. Res. 1989, 15, 355–373. [Google Scholar] [CrossRef]
- Murugesu, S.; Saso, S.; Jones, B.P.; Bracewell-Milnes, T.; Athanasiou, T.; Mania, A.; Serhal, P.; Ben-Nagi, J. Does the Use of Calcium Ionophore during Artificial Oocyte Activation Demonstrate an Effect on Pregnancy Rate? A Meta-Analysis. Fertil. Steril. 2017, 108, 468–482.e3. [Google Scholar] [CrossRef] [PubMed]
- Shoham, Z.; Homburg, R.; Jacobs, H.S. Induction of Ovulation with Pulsatile GnRH. Baillieres. Clin. Obstet. Gynaecol. 1990, 4, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Boelaert, K. Iodine Deficiency and Thyroid Disorders. Lancet Diabetes Endocrinol. 2015, 3, 286–295. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Iron. EFSA J. 2015, 13, 4254. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Calcium. EFSA J. 2015, 13, 4101. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Magnesium. EFSA J. 2015, 13, 4186. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Iodine. EFSA J. 2014, 12, 3660. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Copper. EFSA J. 2015, 13, 4253. [Google Scholar] [CrossRef]
- Institute of Medicine; Food and Nutrition Board; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Subcommittee of Interpretation and Uses of Dietary Reference Intakes; Subcommittee on Upper Reference Levels of Nutrients; Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Cambridge, MA, USA, 2002; ISBN 978-0-30-907279-3. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Manganese. EFSA J. 2013, 11, 3419. [Google Scholar] [CrossRef]
- Obiagwu, H.I.; Eleje, G.U.; Obiechina, N.J.A.; Nwosu, B.O.; Udigwe, G.O.; Ikechebelu, J.I.; Ugboaja, J.O.; Okoro, C.C.; Okonkwo, I.O.; Okwuosa, A.O.; et al. Efficacy of Zinc Supplementation for the Treatment of Dysmenorrhoea: A Double-Blind Randomised Controlled Trial. J. Int. Med. Res. 2023, 51, 3000605231171489. [Google Scholar] [CrossRef]
- Jamilian, M.; Foroozanfard, F.; Bahmani, F.; Talaee, R.; Monavari, M.; Asemi, Z. Effects of Zinc Supplementation on Endocrine Outcomes in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2016, 170, 271–278. [Google Scholar] [CrossRef]
- Afshar Ebrahimi, F.; Foroozanfard, F.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The Effects of Magnesium and Zinc Co-Supplementation on Biomarkers of Inflammation and Oxidative Stress, and Gene Expression Related to Inflammation in Polycystic Ovary Syndrome: A Randomized Controlled Clinical Trial. Biol. Trace Elem. Res. 2018, 184, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Mirhosseini, N.; Eslahi, M.; Bahmani, F.; Shokrpour, M.; Chamani, M.; Asemi, Z. The Effects of Magnesium-Zinc-Calcium-Vitamin D Co-Supplementation on Biomarkers of Inflammation, Oxidative Stress and Pregnancy Outcomes in Gestational Diabetes. BMC Pregnancy Childbirth 2019, 19, 107. [Google Scholar] [CrossRef]
- Jamilian, M.; Sabzevar, N.K.; Asemi, Z. The Effect of Magnesium and Vitamin E Co-Supplementation on Glycemic Control and Markers of Cardio-Metabolic Risk in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Horm. Metab. Res. 2019, 51, 100–105. [Google Scholar]
- Nouri, K.; Walch, K.; Weghofer, A.; Imhof, M.; Egarter, C.; Ott, J. The Impact of a Standardized Oral Multinutrient Supplementation on Embryo Quality in in Vitro Fertilization/Intracytoplasmic Sperm Injection: A Prospective Randomized Trial. Gynecol. Obstet. Investig. 2017, 82, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, R.D.; Aflatoonian, A.; Modarresi, S.; Sekhavat, L.; MohammadTaheri, S. Therapeutic Effects of Calcium & Vitamin D Supplementation in Women with PCOS. Complement. Ther. Clin. Pract. 2012, 18, 85–88. [Google Scholar] [PubMed]
- Rashidi, B.; Haghollahi, F.; Shariat, M.; Zayerii, F. The Effects of Calcium-Vitamin D and Metformin on Polycystic Ovary Syndrome: A Pilot Study. Taiwan J. Obstet. Gynecol. 2009, 48, 142–147. [Google Scholar] [CrossRef]
- Jaripur, M.; Ghasemi-Tehrani, H.; Askari, G.; Gholizadeh-Moghaddam, M.; Clark, C.C.T.; Rouhani, M.H. The Effects of Magnesium Supplementation on Abnormal Uterine Bleeding, Alopecia, Quality of Life, and Acne in Women with Polycystic Ovary Syndrome: A Randomized Clinical Trial. Reprod. Biol. Endocrinol. 2022, 20, 110. [Google Scholar] [CrossRef]
- Rayman, M.P.; Searle, E.; Kelly, L.; Johnsen, S.; Bodman-Smith, K.; Bath, S.C.; Mao, J.; Redman, C.W.G. Effect of Selenium on Markers of Risk of Pre-Eclampsia in UK Pregnant Women: A Randomised, Controlled Pilot Trial. Br. J. Nutr. 2014, 112, 99–111. [Google Scholar] [CrossRef]
- World Health Organization; FAO. Vitamin and Mineral Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2004; ISBN 9789241546126. [Google Scholar]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Institute of Medicine (US). Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Hennigar, S.R.; Lieberman, H.R.; Fulgoni, V.L., 3rd; McClung, J.P. Serum Zinc Concentrations in the US Population Are Related to Sex, Age, and Time of Blood Draw but Not Dietary or Supplemental Zinc. J. Nutr. 2018, 148, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- DeLoughery, T.G. Iron Deficiency Anemia. Med. Clin. N. Am. 2017, 101, 319–332. [Google Scholar] [CrossRef]
- Catharine Ross, A. Modern Nutrition in Health and Disease; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014; ISBN 978-1-60-547461-8. [Google Scholar]
- Institute of Medicine; Food and Nutrition Board; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Cambridge, MA, USA, 1999; ISBN 978-0-30-906403-3. [Google Scholar]
- Bo, S.; Durazzo, M.; Gambino, R.; Berutti, C.; Milanesio, N.; Caropreso, A.; Gentile, L.; Cassader, M.; Cavallo-Perin, P.; Pagano, G. Associations of Dietary and Serum Copper with Inflammation, Oxidative Stress, and Metabolic Variables in Adults. J. Nutr. 2008, 138, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Ambroziak, U.; Hybsier, S.; Shahnazaryan, U.; Krasnodębska-Kiljańska, M.; Rijntjes, E.; Bartoszewicz, Z.; Bednarczuk, T.; Schomburg, L. Severe Selenium Deficits in Pregnant Women Irrespective of Autoimmune Thyroid Disease in an Area with Marginal Selenium Intake. J. Trace Elem. Med. Biol. 2017, 44, 186–191. [Google Scholar] [CrossRef]
- Tulić, L.; Vidaković, S.; Tulić, I.; Ćurčić, M.; Bulat, Z. Toxic Metal and Trace Element Concentrations in Blood and Outcome of In Vitro Fertilization in Women. Biol. Trace Elem. Res. 2019, 188, 284–294. [Google Scholar] [CrossRef]
- Pitkin, R.M.; Gebhardt, M.P. Serum Calcium Concentrations in Human Pregnancy. Am. J. Obstet. Gynecol. 1977, 127, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Polzikov, M.; Blinov, D.; Barakhoeva, Z.; Vovk, L.; Fetisova, Y.; Ovchinnikova, M.; Tischenko, M.; Zorina, I.; Yurasov, V.; Ushakova, T.; et al. Association of the Serum Folate and Total Calcium and Magnesium Levels Before Ovarian Stimulation With Outcomes of Fresh Fertilization Cycles in Normogonadotropic Women. Front. Endocrinol. 2022, 13, 732731. [Google Scholar] [CrossRef]
- Kurzel, R.B. Serum Magnesium Levels in Pregnancy and Preterm Labor. Am. J. Perinatol. 1991, 8, 119–127. [Google Scholar] [CrossRef]
- Pan, Z.; Cui, T.; Chen, W.; Gao, S.; Pearce, E.N.; Wang, W.; Chen, Y.; Guo, W.; Tan, L.; Shen, J.; et al. Serum Iodine Concentration in Pregnant Women and Its Association with Urinary Iodine Concentration and Thyroid Function. Clin. Endocrinol. 2019, 90, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.G.; Andersson, M.; Hunziker, S.; Zimmermann, M.B.; Moore, S.E. Effects of an Iodine-Containing Prenatal Multiple Micronutrient on Maternal and Infant Iodine Status and Thyroid Function: A Randomized Trial in The Gambia. Thyroid 2020, 30, 1355–1365. [Google Scholar] [CrossRef] [PubMed]


| Mineral | Zn | Fe | Se | Ca | Mg | I | Cu | Mn |
|---|---|---|---|---|---|---|---|---|
| Recommended daily intake | 8–11 mg [398] | 18–27 mg [399] | 55–60 µg [400] | 1000–1200 mg [401] | 320–350 mg [402] | 70–130 µg [403] | 1.3 mg [404] | 3 mg [405,406] |
| Daily intake in studies | 40 mg [407]–50 mg [72,408,409] | 60 mg [102] 100 mg [410]–250 mg [410,411] | 70 µg [412] | 1000 mg [413,414] | 250 mg [409,415] | 60 μg [416] | 1 mg [102] | 3 mg |
| Main food source | Meat, dairy, nuts, whole grains [405] | Leafy Greens, Meat, Legumes [417] | Brazil nuts, fish, whole-wheat bread [418] | Dairy products, fortified foods, leafy greens [419] | Green leafy vegetables, nuts, seeds, whole grains | Brazil nuts, fish, whole-wheat bread [418] | Oyster, nuts, sunflower seeds, liver, whole grain, dark chocolate | Brown rice, hazelnuts, chickpeas, spinach, pumpkin seeds |
| Recommended serum levels | 70–120 µg/dL [420] | 15–150 ng/mL [421] | 70–150 ng/mL [422] | 8.6–10.2 mg/dL [419] | 1.7–2.2 mg/dL [423] | 100 µg/L (Urine) | 70–150 µg/dL [424] | 4–15 µg/L [405] |
| Optimal serum levels in studies | ≥56 μg/dL [78] | N.A. | 45 μg/L [425]–109 μg/L [426] | 8.8–10.4 mg/dL [427] and <94 mg/L [428] | >1.4 mg/dL [429] 20.7 mg/L [428] 2.22–3.48 mg/dL [426] | 79.9–138.5 μg/L [430] and >150 μg/L (urinary) [431] | 88–177 μg/dL [405,426] | N.A. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapper, C.; Oppelt, P.; Ganhör, C.; Gyunesh, A.A.; Arbeithuber, B.; Stelzl, P.; Rezk-Füreder, M. Minerals and the Menstrual Cycle: Impacts on Ovulation and Endometrial Health. Nutrients 2024, 16, 1008. https://doi.org/10.3390/nu16071008
Kapper C, Oppelt P, Ganhör C, Gyunesh AA, Arbeithuber B, Stelzl P, Rezk-Füreder M. Minerals and the Menstrual Cycle: Impacts on Ovulation and Endometrial Health. Nutrients. 2024; 16(7):1008. https://doi.org/10.3390/nu16071008
Chicago/Turabian StyleKapper, Celine, Peter Oppelt, Clara Ganhör, Ayberk Alp Gyunesh, Barbara Arbeithuber, Patrick Stelzl, and Marlene Rezk-Füreder. 2024. "Minerals and the Menstrual Cycle: Impacts on Ovulation and Endometrial Health" Nutrients 16, no. 7: 1008. https://doi.org/10.3390/nu16071008