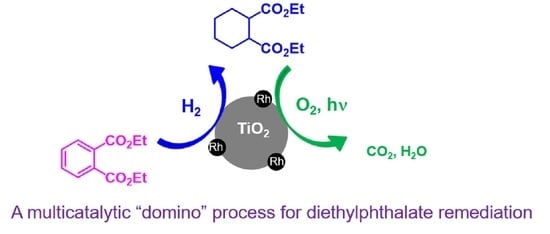
Remediation of Diethyl Phthalate in Aqueous Effluents with TiO2-Supported Rh0 Nanoparticles as Multicatalytic Materials
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Preparation of Metal NPs@TiO2
3.3. General Procedure for Hydrogenation under Atmospheric Hydrogen Pressure
3.4. General Procedure for Hydrogenation under Hydrogen Pressure
3.5. Photocatalytic Experiments
3.6. Adsorption Experiments
3.7. Transmission Electron Microscopy (TEM) Analysis
3.8. X-ray Photoelectron Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Hens, A.; Aguilar-Caballos, M. Social and economic interest in the control of phthalic acid esters. TrAC Trends Anal. Chem. 2003, 22, 847–857. [Google Scholar] [CrossRef]
- Prasad, B. Phthalate pollution: Environmental fate and cumulative human exposure index using the multivariate analysis approach. Environ. Sci. Process. Impacts 2021, 23, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Bergé, A.; Cladière, M.; Gasperi, J.; Coursimault, A.; Tassin, B.; Moilleron, R. Meta-analysis of environmental contamination by phthalates. Environ. Sci. Pollut. Res. 2013, 20, 8057–8076. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, J.; Zheng, C.; Zhang, X.; Zhang, A.; Qi, H. Phthalate pollution driven by the industrial plastics market: A case study of the plastic market in Yuyao City, China. Environ. Sci. Pollut. Res. 2019, 26, 11224–11233. [Google Scholar] [CrossRef]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environmental fate of phthalate esters: A literature review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Jobling, S.; Reynolds, T.; White, R.; Parker, M.G.; Sumpter, J.P. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ. Health Perspect. 1995, 103, 582–587. [Google Scholar] [CrossRef]
- Van Wezel, A.; Van Vlaardingen, P.; Posthumus, R.; Crommentuijn, G.; Sijm, D. Environmental Risk Limits for Two Phthalates, with Special Emphasis on Endocrine Disruptive Properties. Ecotoxicol. Environ. Saf. 2000, 46, 305–321. [Google Scholar] [CrossRef]
- Wittassek, M.; Angerer, J.; Kolossa-Gehring, M.; Schäfer, S.D.; Klockenbusch, W.; Dobler, L.; Günsel, A.K.; Müller, A.; Wiesmüller, G.A. Fetal exposure to phthalates—A pilot study. Int. J. Hyg. Environ. Health 2009, 212, 492–498. [Google Scholar] [CrossRef]
- Peng, X.; Feng, L.; Li, X. Pathway of diethyl phthalate photolysis in sea-water determined by gas chromatography–mass spectrometry and compound-specific isotope analysis. Chemosphere 2013, 90, 220–226. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, T.; Fang, H.H.P.; He, J. Phthalates biodegradation in the environment. Appl. Microbiol. Biotechnol. 2008, 80, 183–198. [Google Scholar] [CrossRef]
- Perpetuo, E.A.; da Silva, E.C.N.; Karolski, B.; do Nascimento, C.A.O. Biodegradation of diethyl-phthalate (DEP) by halo-tolerant bacteria isolated from an estuarine environment. Biodegradation 2020, 31, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Shaida, M.A.; Dutta, R.; Sen, A. Removal of diethyl phthalate via adsorption on mineral rich waste coal modified with chitosan. J. Mol. Liq. 2018, 261, 271–282. [Google Scholar] [CrossRef]
- Alves, D.D.S.; Healy, B.; Pinto, L.; Cadaval, T.; Breslin, C. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- Guo, R.; Yan, L.; Rao, P.; Wang, R.; Guo, X. Nitrogen and sulfur co-doped biochar derived from peanut shell with en-hanced adsorption capacity for diethyl phthalate. Environ. Pollut. 2020, 258, 113674. [Google Scholar] [CrossRef]
- Cesaro, V.; Belgiorno, V. Removal of Endocrine Disruptors from Urban Wastewater by Advanced Oxidation Processes (AOPs): A Review. Open Biotechnol. J. 2016, 10, 151–172. [Google Scholar] [CrossRef]
- Mansouri, L.; Sabelfeld, M.; Geissen, S.-U.; Bousselmi, L. Catalytic ozonation of model organic compounds in aqueous solution promoted by metallic oxides. Desalin. Water Treat. 2015, 53, 1089–1100. [Google Scholar] [CrossRef]
- Zammit, I.; Vaiano, V.; Ribeiro, A.R.; Silva, A.M.T.; Manaia, C.M.; Rizzo, L. Immobilised Cerium-Doped Zinc Oxide as a Photocatalyst for the Degradation of Antibiotics and the Inactivation of Antibiotic-Resistant Bacteria. Catalysts 2019, 9, 222. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Zhang, T.; Fang, H.H. Anaerobic degradation of dimethyl phthalate in wastewater in a UASB reactor. Water Res. 2007, 41, 2879–2884. [Google Scholar] [CrossRef]
- Roslev, P.; Vorkamp, K.; Aarup, J.; Frederiksen, K.; Nielsen, P.H. Degradation of phthalate esters in an activated sludge wastewater treatment plant. Water Res. 2007, 41, 969–976. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.A.; Ocampo-Pérez, R.; Leyva-Ramos, R.; Sanchez-Polo, M.; Rivera-Utrilla, J.; Méndez-Díaz, J.D. Removal of diethyl phthalate from water solution by adsorption, photo-oxidation, ozonation and advanced oxidation process (UV/H2O2, O3/H2O2 and O3/activated carbon). Sci. Total Environ. 2013, 442, 26–35. [Google Scholar] [CrossRef]
- Mansouri, L.; Tizaoui, C.; Geissen, S.-U.; Bousselmi, L. A comparative study on ozone, hydrogen peroxide and UV based advanced oxidation processes for efficient removal of diethyl phthalate in water. J. Hazard. Mater. 2019, 363, 401–411. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Q.; Chen, Y.; Tian, Q.; Zhao, G. Efficient removal of dimethyl phthalate with activated iron-doped carbon aerogel through an integrated adsorption and electro-Fenton oxidation process. Carbon 2017, 124, 111–122. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.; Dionysiou, D.D.; Liu, C.; Tong, Y.; Gao, J.; Fang, G.; Zhou, D. Efficient activation of peroxymonosulfate by copper sulfide for diethyl phthalate degradation: Performance, radical generation and mechanism. Sci. Total. Environ. 2020, 749, 142387. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gao, N.-Y.; Sun, X.-F.; Xia, S.; Rui, M.; Simonnot, M.-O.; Causserand, C.; Zhao, J.-F. Photochemical degradation of diethyl phthalate with UV/H2O2. J. Hazard. Mater. 2007, 139, 132–139. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.F.; Cagnon, B.; Chedeville, O.; Fauduet, H. Removal of a mix of endocrine disrupters from different natural matrices by ozone/activated carbon coupling process. Desalination Water Treat. 2013, 52, 4395–4403. [Google Scholar] [CrossRef]
- Mohan, S.; Mamane, H.; Avisar, D.; Gozlan, I.; Kaplan, A.; Dayalan, G. Treatment of Diethyl Phthalate Leached from Plastic Products in Municipal Solid Waste Using an Ozone-Based Advanced Oxidation Process. Materials 2019, 12, 4119. [Google Scholar] [CrossRef]
- Wen, G.; Wang, S.-J.; Ma, J.; Huang, T.-L.; Liu, Z.-Q.; Zhao, L.; Su, J.-F. Enhanced ozonation degradation of di-n-butyl phthalate by zero-valent zinc in aqueous solution: Performance and mechanism. J. Hazard. Mater. 2014, 265, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, J.; Wang, C.; Yang, S.; Zhu, W. Catalytic ozonation of dimethyl phthalate with RuO2/Al2O3 catalysts prepared by microwave irradiation. Catal. Commun. 2013, 41, 1–5. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Rodríguez, J.L.; Poznyak, T.; Chairez, I.; Dueñas, J. Catalytic effect of γ-Al(OH)3, α-FeOOH, and α-Fe2O3 on the ozonation-based decomposition of diethyl phthalate adsorbed on sand and soil. Environ. Sci. Pollut. Res. 2021, 28, 974–981. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Mohamed, A.R.; Lee, K.T. Degrading endocrine disrupting chemicals from wastewater by TiO2 pho-tocatalysis: A review. Int. J. Photoenergy 2012, 2012, 185159. [Google Scholar] [CrossRef] [Green Version]
- Al-Madanat, O.; AlSalka, Y.; Ramadan, W.; Bahnemann, D. TiO2 Photocatalysis for the Transformation of Aromatic Water Pollutants into Fuels. Catalysts 2021, 11, 317. [Google Scholar] [CrossRef]
- Mashuri, S.I.S.; Ibrahim, M.L.; Kasim, M.F.; Mastuli, M.S.; Rashid, U.; Abdullah, A.H.; Islam, A.; Mijan, N.A.; Tan, Y.H.; Mansir, N.; et al. Photocatalysis for Organic Wastewater Treatment: From the Basis to Current Challenges for Society. Catalysts 2020, 10, 1260. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Muneer, M.; Theurich, J.; Bahnemann, D.B.D. Titanium dioxide mediated photocatalytic degradation of 1,2-diethyl phthalate. J. Photochem. Photobiol. A Chem. 2001, 143, 213–219. [Google Scholar] [CrossRef]
- Huang, W.-B.; Chen, C.-Y. Photocatalytic Degradation of Diethyl Phthalate (DEP) in Water Using TiO2. Water Air Soil Pollut. 2009, 207, 349–355. [Google Scholar] [CrossRef]
- Ding, X.; An, T.; Li, G.; Chen, J.; Sheng, G.; Fu, J.; Zhao, J. Photocatalytic degradation of dimethyl phthalate ester using nov-el hydrophobic TiO2 pillared montmorillonite photocatalyst. Res. Chem. Intermediat. 2008, 34, 67–83. [Google Scholar] [CrossRef]
- Chang, C.-F.; Man, C.-Y. Titania-Coated Magnetic Composites as Photocatalysts for Phthalate Photodegradation. Ind. Eng. Chem. Res. 2011, 50, 11620–11627. [Google Scholar] [CrossRef]
- Chalasani, R.; Vasudevan, S. Cyclodextrin-Functionalized Fe3O4@TiO2: Reusable, Magnetic Nanoparticles for Photocatalytic Degradation of Endocrine-Disrupting Chemicals in Water Supplies. ACS Nano 2013, 7, 4093–4104. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, H.; Shi, W.; Xu, Y.; Wu, D. Enhanced visible photocatalytic activity of titania–silica photocatalysts: Effect of carbon and silver doping. Catal. Sci. Technol. 2012, 2, 1213–1220. [Google Scholar] [CrossRef]
- Singla, P.; Pandey, O.P.; Singh, K. Study of photocatalytic degradation of environmentally harmful phthalate esters using Ni-doped TiO2 nanoparticles. Int. J. Environ. Sci. Technol. 2015, 13, 849–856. [Google Scholar] [CrossRef] [Green Version]
- Ki, S.J.; Park, Y.-K.; Kim, J.-S.; Lee, W.-J.; Lee, H.; Jung, S.-C. Facile preparation of tungsten oxide doped TiO2 photocatalysts using liquid phase plasma process for enhanced degradation of diethyl phthalate. Chem. Eng. J. 2019, 377, 120087. [Google Scholar] [CrossRef]
- Narayan, N.; Meiyazhagan, A.; Vajtai, R. Metal Nanoparticles as Green Catalysts. Materials 2019, 12, 3602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denicourt-Nowicki, A.; Roucoux, A. Odyssey in Polyphasic Catalysis by Metal Nanoparticles. Chem. Rec. 2016, 16, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Denicourt-Nowicki, A.; Mordvinova, N.; Roucoux, A. Metal nanoparticles in water: A relevant toolbox for green catalysis. In Nanoparticles in Catalysis. Advances in Synthesis and Applications; Philippot, K., Roucoux, A., Eds.; Wiley-VCH: Weinheim, Germany, 2021; pp. 45–71. [Google Scholar]
- Hubert, C.; Denicourt-Nowicki, A.; Beaunier, P.; Roucoux, A. TiO2-supported Rh nanoparticles: From green catalyst preparation to application in arene hydrogenation in neat water. Green Chem. 2010, 12, 1167–1170. [Google Scholar] [CrossRef]
- Heuson, E.; Dumeignil, F. The various levels of integration of chemo- and bio-catalysis towards hybrid catalysis. Catal. Sci. Technol. 2020, 10, 7082–7100. [Google Scholar] [CrossRef]
- Hubert, C.; Bilé, E.G.; Denicourt-Nowicki, A.; Roucoux, A. Tandem dehalogenation–hydrogenation reaction of halogenoarenes as model substrates of endocrine disruptors in water: Rhodium nanoparticles in suspension vs. on silica support. Appl. Catal. A Gen. 2011, 394, 215–219. [Google Scholar] [CrossRef]
- Tan, B.L.; Hawker, D.W.; Müller, J.F.; Leusch, F.; Tremblay, L.; Chapman, H.F. Modelling of the fate of selected endocrine disruptors in a municipal wastewater treatment plant in South East Queensland, Australia. Chemosphere 2007, 69, 644–654. [Google Scholar] [CrossRef]
- Tan, G.H. Residue levels of phthalate esters in water and sediment samples from the Klang River basin. Bull. Environ. Contam. Toxicol. 1995, 54, 171–176. [Google Scholar] [CrossRef]
- Staples, C.A.; Parkerton, T.F.; Peterson, D.R. A risk assessment of selected phthalate esters in North American and West-ern European surface waters. Chemosphere 2000, 40, 885–891. [Google Scholar] [CrossRef]
- Gani, K.M.; Tyagi, V.K.; Kazmi, A.A. Occurrence of phthalates in aquatic environment and their removal during wastewater treatment processes: A review. Environ. Sci. Pollut. Res. 2017, 24, 17267–17284. [Google Scholar] [CrossRef]
- Hubert, C.; Bilé, E.G.; Denicourt-Nowicki, A.; Roucoux, A. Rh(0) colloids supported on TiO2: A highly active and pertinent tandem in neat water for the hydrogenation of aromatics. Green Chem. 2011, 13, 1766–1771. [Google Scholar] [CrossRef]
- Bilé, E.G.; Sassine, R.; Denicourt-Nowicki, A.; Launay, F.; Roucoux, A. New ammonium surfactant-stabilized rho-dium(0) colloidal suspensions: Influence of novel counter-anions on physico-chemical and catalytic properties. Dalton Trans. 2011, 40, 6524–6531. [Google Scholar] [CrossRef]
- Barthe, L.; Hemati, M.; Philippot, K.; Chaudret, B.; Denicourt-nowicki, A.; Roucoux, A. Rhodium colloidal suspension deposition on porous silica particles by dry impregnation: Study of the influence of the reaction conditions on nanoparticles loca-tion and dispersion and catalytic reactivity. Chem. Eng. J. 2009, 151, 372–379. [Google Scholar] [CrossRef]
- Pélisson, C.-H.; Hubert, C.; Denicourt-Nowicki, A.; Roucoux, A. From Hydroxyalkylammonium Salts to Protected-Rh(0) Nanoparticles for Catalysis in Water: Comparative Studies of the Polar Heads. Top. Catal. 2013, 56, 1220–1227. [Google Scholar] [CrossRef]
- Pouilleau, J.; Devilliers, D.; Groult, H.; Marcus, P. Surface study of a titanium-based ceramic electrode material by X-ray photoelectron spectroscopy. J. Mater. Sci. 1997, 32, 5645–5651. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Erdem, B.; Hunsicker, R.A.; Simmons, G.W.; Sudol, E.D.; Dimonie, V.L.; El-Aasser, M.S. XPS and FTIR Surface Characterization of TiO2 Particles Used in Polymer Encapsulation. Langmuir 2001, 17, 2664–2669. [Google Scholar] [CrossRef]
- Naya, S.-I.; Nikawa, T.; Kimura, K.; Tada, H. Rapid and Complete Removal of Nonylphenol by Gold Nanoparticle/Rutile Titanium(IV) Oxide Plasmon Photocatalyst. ACS Catal. 2013, 3, 903–907. [Google Scholar] [CrossRef]
- Pugazhenthiran, N.; Murugesan, S.; Sathishkumar, P.; Anandan, S. Photocatalytic degradation of ceftiofur sodium in the presence of gold nanoparticles loaded TiO2 under UV–visible light. Chem. Eng. J. 2014, 241, 401–409. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmen-tal applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Ahmad, M.; Nisar, A.; Sun, H.; Karim, S.; Khan, M.; Khan, S.D.; Iqbal, M.; Hussain, S.Z. Enhanced photocatalytic and electrochemical properties of Au nanoparticles supported TiO2 microspheres. New J. Chem. 2014, 38, 1424–1432. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 Reduction with H2O on TiO2 Nanocrystals: Comparison of Anatase, Rutile, and Brookite Polymorphs and Exploration of Surface Chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.A.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakawa, T.; Kamat, P.V. Charge Separation and Catalytic Activity of Ag@TiO2 Core—Shell Composite Clusters under UV—Irradiation. J. Am. Chem. Soc. 2005, 127, 3928–3934. [Google Scholar] [CrossRef]
- Ji, Z.; Ismail, M.N.; Callahan, D.M.; Pandowo, E.; Cai, Z.; Goodrich, T.L.; Ziemer, K.S.; Warzywoda, J.; Sacco, A. The role of silver nanoparticles on silver modified titanosilicate ETS-10 in visible light photocatalysis. Appl. Catal. B Environ. 2011, 102, 323–333. [Google Scholar] [CrossRef]
- Chacon, G.; Dupont, J. Arene Hydrogenation by Metal Nanoparticles in Ionic Liquids. ChemCatChem 2019, 11, 333–341. [Google Scholar] [CrossRef]
- Schulz, J.; Roucoux, A.; Patin, H. Stabilized Rhodium(0) Nanoparticles: A Reusable Hydrogenation Catalyst for Arene Derivatives in a Biphasic Water-Liquid System. Chem. Eur. J. 2000, 6, 618–624. [Google Scholar] [CrossRef]






| Entry | H2 Pressure (Bar) | t (h) | TOF 2 (h−1) |
|---|---|---|---|
| 1 3 | 40 | 0.18 | 1670 |
| 2 | 30 | 0.18 | 1670 |
| 3 | 1 | 3.5 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denicourt-Nowicki, A.; Pélisson, C.-H.; Soutrel, I.; Favier, L.; Roucoux, A. Remediation of Diethyl Phthalate in Aqueous Effluents with TiO2-Supported Rh0 Nanoparticles as Multicatalytic Materials. Catalysts 2021, 11, 1166. https://doi.org/10.3390/catal11101166
Denicourt-Nowicki A, Pélisson C-H, Soutrel I, Favier L, Roucoux A. Remediation of Diethyl Phthalate in Aqueous Effluents with TiO2-Supported Rh0 Nanoparticles as Multicatalytic Materials. Catalysts. 2021; 11(10):1166. https://doi.org/10.3390/catal11101166
Chicago/Turabian StyleDenicourt-Nowicki, Audrey, Carl-Hugo Pélisson, Isabelle Soutrel, Lidia Favier, and Alain Roucoux. 2021. "Remediation of Diethyl Phthalate in Aqueous Effluents with TiO2-Supported Rh0 Nanoparticles as Multicatalytic Materials" Catalysts 11, no. 10: 1166. https://doi.org/10.3390/catal11101166