Advancements in Fermented Beverage Safety: Isolation and Application of Clavispora lusitaniae Cl-p for Ethyl Carbamate Degradation and Enhanced Flavor Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Culture Medium
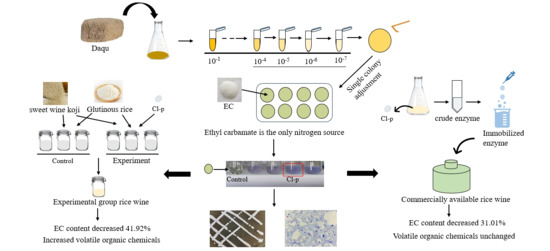
2.3. Screening of Ethyl Carbamate-Degrading Strains
2.4. Identification of Strains
2.5. Analysis on the Tolerance and Fermentation Performance of the Strain
2.6. Application of Cl-p Strain in Rice Wine Fermentation
2.7. Application of Cl-p Strain Crude Enzyme in Finished Yellow Rice Wine
3. Results and Discussion
3.1. Isolation and Screening of Ethyl Carbamate-Degrading Strains
3.2. Identification of Cl-p Strain
3.3. Analysis of Tolerance and Fermentation Performance of Cl-p Strain
3.4. The Utilization of the Cl-p Strain in the Fermentation Process of Rice Wine
3.4.1. Contribution of Cl-p Strain to Decrease in EC Content in Rice Wine Fermentation
3.4.2. Effect of Cl-p Strain on Flavor Substances of Rice Wine
3.5. Application of Cl-p Strain Crude Enzyme in Finished Rice Wine
3.5.1. Degradation of EC in Finished Yellow Rice Wine by Crude Enzyme of Cl-p Strain
3.5.2. Influence of Cl-p Strain Crude Enzyme on Flavor Substances of Finished Yellow Rice Wine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, M.-L.; Liu, J.; Chen, Q.-H.; Liu, X.-J.; He, G.-Q.; Chen, J.-C. Determination of ethyl carbamate in Chinese yellow rice wine using high-performance liquid chromatography with fluorescence detection. Int. J. Food Sci. Technol. 2010, 45, 1297–1302. [Google Scholar] [CrossRef]
- Hasnip, S.; Crews, C.; Potter, N.; Christy, J.; Chan, D.; Bondu, T.; Matthews, W.; Walters, B.; Patel, K. Survey of ethyl carbamate in fermented foods sold in the United Kingdom in 2004. J. Agric. Food Chem. 2007, 55, 2755–2759. [Google Scholar] [CrossRef] [PubMed]
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Cogliano, V. Carcinogenicity of alcoholic beverages. Lancet. Oncol. 2007, 8, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, K.; Takebe, S.; Sakai, T. Urethane-hydrolyzing enzyme from Citrobacter sp. Chem. Pharm. Bull. 1990, 38, 1326–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.J.; Imamura, L.; Kobashi, K. Urethanase of Bacillus licheniformis sp. isolated from mouse gastrointestine. Chem. Pharm. Bull. 1991, 39, 3303–3306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.J.; Kobashi, K. Purification and characterization of iron-containing urethanase from Bacillus licheniformis. Biol. Pharm. Bull. 1994, 17, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, B.R.; Bapuji, M. Characterization of urethanase from Micrococcus species associated with the marine sponge (Spirasfrella species). Lett. Appl. Microbiol. 1997, 25, 393–396. [Google Scholar] [CrossRef]
- Akutsu-Shigeno, Y.; Adachi, Y.; Yamada, C.; Toyoshima, K.; Nomura, N.; Uchiyama, H.; Nakajima-Kambe, T. Isolation of a bacterium that degrades urethane compounds and characterization of its urethane hydrolase. Appl. Microbiol. Biotechnol. 2006, 70, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, Q.; Xu, Y.; Zhao, G. Study on Rhodotorula mucilaginosa and its degradation of ethyl carbamate. Ind. Microbiol. 2013, 43, 44–48. [Google Scholar]
- Zhou, N.; Gu, X.; Tian, Y. Isolation and characterization of urethanase from Penicillium variabile and its application to reduce ethyl carbamate contamination in Chinese rice wine. Appl. Biochem. Biotechnol. 2013, 170, 718–728. [Google Scholar] [CrossRef]
- Bu, P.P.; Chen, J.; Du, G. Isolation, purification and enzymatic properties of salt-tolerant ethyl carbamate hydrolase. Chin. J. Biotechnol. 2014, 30, 404–411. [Google Scholar] [CrossRef]
- Li, J.; Fang, F.; Jiran, Z.; Long, L.; Guocheng, D.; Jian, C. Purification and enzymatic properties of ethyl carbamate hydrolase. J. Food Sci. Biotechnol. 2014, 33, 1239–1245. [Google Scholar]
- Mohapatra, B.R. An Insight into the Prevalence and Enzymatic Abatement of Urethane in Fermented Beverages. In Microbial Biotechnology: Volume 2. Application in Food and Pharmacology; Patra, J.K., Das, G., Shin, H.-S., Eds.; Springer: Singapore, 2018; pp. 153–170. [Google Scholar] [CrossRef]
- Thongekkaew, J.; Fujii, T.; Masaki, K. Ethyl Carbamate Degrading Enzyme from Yeast Meyerozyma caribbica strain SKa5: Purification and Biochemical Properties. Rep. Compr. Res. Inst. Liquor 2018, 9, 124–132. [Google Scholar]
- Masaki, K.; Mizukure, T.; Kakizono, D.; Fujihara, K.; Fujii, T.; Mukai, N. New urethanase from the yeast Candida parapsilosis. J. Biosci. Bioeng. 2020, 130, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Masaki, K.; Fujihara, K.; Kakizono, D.; Mizukure, T.; Okuda, M.; Mukai, N. Aspergillus oryzae acetamidase catalyzes degradation of ethyl carbamate. J. Biosci. Bioeng. 2020, 130, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Kang, T. Screening, Expression and Activity Modification of Ethyl Carbamate Hydrolase. Master’s Thesis, Zhejiang University, Hangzhou, China, 2021. [Google Scholar]
- Zheng, H.; Meng, K.; Liu, J.; Lin, Z.; Peng, Q.; Xie, G.; Wu, P.; Elsheery, N.I. Identification and expression of bifunctional acid urea-degrading enzyme/urethanase from Enterobacter sp. R-SYB082 and its application in degradation of ethyl carbamate in Chinese rice wine (Huangjiu). J. Sci. Food Agric. 2022, 102, 4599–4608. [Google Scholar] [CrossRef]
- Arévalo-Jaimes, B.V.; Admella, J.; Blanco-Cabra, N.; Torrents, E. Culture media influences Candida parapsilosis growth, susceptibility, and virulence. Front. Cell. Infect. Microbiol. 2023, 13, 1323619. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Q.; Tian, Y. The Invention Discloses a Yeast for Producing Flavoring Wine and Its Use and Method. CN111363686B, 28 September 2021. [Google Scholar]
- Jiang, G.; Liu, Y.; Gu, Y.; Lin, Y.; Cao, Y.; Wang, X.; Shan, C. Candida Parapsilosis and Traditional Chinese Medicine Microecological Preparation for Preventing and Treating Ruminant Diarrhea, and Preparation Method Thereof. CN116162557B, 1 February 2023. [Google Scholar]
- Shen, P. Screening and Technological Study on Local Saccharomyces Cerevisiae Capable of Degrading Ethyl Carbamate. Master’s Thesis, Ningxia University, Yinchuan, China, 2021. [Google Scholar]
- Zou, M.Y.; Xingui, Z.; Qixing, S.; Lichen, H. Identification of a yeast producing 2-phenylethanol and its application in soy sauce brewing. China Brew. 2020, 39, 173–178. [Google Scholar]
- QB/T 4257-2011; General Analytical Method for Distiller’s Daqu. Ministry of Industry and Information Technology: Beijing, China, 2011.
- Gui, J. Processing Technology Optimization and Product Flavor Analysis of Jiangxi Rice Wine. Master’ Thesis, Nanchang University, Nanchang, China, 2023. [Google Scholar]
- GB/T 18204.2-2014; Hygiene Inspection Methods for Public Places. Part 2: Chemical Pollutants. Standardization Administration of China: Beijing, China, 2014.
- Mo, W.-M.; He, H.-L.; Xu, X.-M.; Huang, B.-F.; Ren, Y.-P. Simultaneous determination of ethyl carbamate, chloropropanols and acrylamide in fermented products, flavoring and related foods by gas chromatography–triple quadrupole mass spectrometry. Food Control 2014, 43, 251–257. [Google Scholar] [CrossRef]
- De Melo Abreu, S.; Alves, A.; Oliveira, B.; Herbert, P. Determination of ethyl carbamate in alcoholic beverages: An interlaboratory study to compare HPLC-FLD with GC-MS methods. Anal. Bioanal. Chem. 2005, 382, 498–503. [Google Scholar] [CrossRef]
- Zhang, Y.; Shaoping, N.; Cheng, W.; Yongming, X. Immobilization of β-D-fructofuranosidase produced by Aspergillus japonicus. J. Nanchang Univ. (Nat. Sci.) 2010, 34, 243–248. [Google Scholar]
- Liu, Q. Expression and Molecular Modification of Bacillus Paralicheniformis Acid Urease and Its Application in Reduction of Ethyl Carbamate in Rice Wine. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2019. [Google Scholar]
- Ding, X.; Qiaoyu, L.; Fan, L.; Weizu, Z.; Jian, C.; Guocheng, D.; Fang, F. Screening of ethyl carbamate degrading strains from Luzhou-flavor liquor and study on enzyme production characteristics. Food Ferment. Ind. 2018, 44, 29–36. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, C.; Ran, G.; Jianmin, Z. Study on UV mutagenesis selection and fermentation conditions of Saccharomyces cerevisiae. Hubei Agric. Sci. 2015, 54, 1664–1667. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiang, S. Determination conditions and orthogonal optimization of traditional Daqu liquefaction power. Liquor Mak. 2012, 39, 71–73. [Google Scholar]
- QB/T 5188-2017; Fermented Red Yeast. Ministry of Industry and Information Technology: Beijing, China, 2017.
- Fan, G.; Liu, P.; Chang, X.; Yin, H.; Cheng, L.; Teng, C.; Gong, Y.; Li, X. Isolation and Identification of a High-Yield Ethyl Caproate-Producing Yeast from Daqu and Optimization of Its Fermentation. Front. Microbiol. 2021, 12, 663744. [Google Scholar] [CrossRef]
- Chen, D.; Ren, Y.; Zhong, Q.; Shao, Y.; Zhao, Y.; Wu, Y. Ethyl carbamate in alcoholic beverages from China: Levels, dietary intake, and risk assessment. Food Control 2017, 72, 283–288. [Google Scholar] [CrossRef]
- Yang, G. Screening of Acid Urease and Aminoethyl Ester Degrading Enzyme Producing Bacteria and Enzyme Characteristics. Master’ Thesis, Jiangnan University, Wuxi, China, 2014. [Google Scholar]
- Fang, R. Research on the Metabolic Patterns and Inhibition Methods of Ethyl Carbamate Production in Traditional Yellow Rice Wine Fermentation. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2017. [Google Scholar]
- Yu, W.; Xie, G.; Wu, D.; Li, X.; Li, J. A Lactobacillus brevis strain with citrulline re-uptake activity for citrulline and ethyl carbamate control during Chinese rice wine fermentation. Food Biosci. 2020, 36, 100612. [Google Scholar] [CrossRef]
- Jia, X.; Zheng, W.; Sun, L.; Chen, X. A Rhizopus oryzae strain Capable of Degrading Ethyl Carbamate and Its Application. CN112592839B, 19 August 2022. [Google Scholar]
- Yu, S.; Zhou, J.; Tian, S.; Zeng, W.; Chen, J.; Du, G.; Xia, X. A Strain for Reducing the Accumulation of Urea and Ethyl Carbamate in Yellow Wine and Its Application. CN113913314B, 24 February 2023. [Google Scholar]
- Ming, H.; Jian, Z.; Mengen, C.; Zhi, G.; Xia, Y.; Yumeng, L. Optimization of fermentation conditions for producing phenylethanol from abnormal Wickham yeast in Daqu. Hubei Agric. Sci. 2015, 54, 3492–3496. [Google Scholar] [CrossRef]
- Wang, Q. Screening of Abnormal Wickham Yeast and Its Effect on Flavor of Rice Wine Fermentation. Master’ Thesis, Wuhan Polytechnic University, Wuhan, China, 2022. [Google Scholar]
- Yue, M.; Gu, C.; Ding, X.; Liu, Y.; Wang, L.; Sun, L. Improvement of the quality of steamed buns by combined fermentation of abnormal Wickham yeast and saccharomyces cerevisiae. Food Sci. 2023, 1–16. [Google Scholar]
- Wang, J.; Yan, C.; Ma, C.; Chang, X.; Li, Z.; Chen, X.; Li, X. Study on interaction between two non-saccharomyces cerevisiae and Aspergillus oryzae. Sci. Technol. Food Ind. 2023, 44, 171–180. [Google Scholar] [CrossRef]
- Wang, C.; Tang, J.; Qiu, S. Profiling of Fungal Diversity and Fermentative Yeasts in Traditional Chinese Xiaoqu. Front. Microbiol. 2020, 11, 554870. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Xue, S.; Guo, H.; Xiong, K.; Lin, X.; Liang, H.; Ji, C.; Huang, Z.; Zhang, S. Genetic Engineering Production of Ethyl Carbamate Hydrolase and Its Application in Degrading Ethyl Carbamate in Chinese Liquor. Foods 2022, 11, 937. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, Y.; Zhou, Z.; Lu, W.; Zhu, Y. Analysis on the types, composition and sources of flavor substances in rice rice wine. Jiangsu Condiment Subsid. Food 2010, 27, 27–29. [Google Scholar] [CrossRef]
- Bao, Z.; Xu, R. Analysis of aroma components of yellow rice wine. Liquor-Mak. Sci. Technol. 1999, 5, 54–55+53. [Google Scholar]
- Li, H.; Feng, T. The key flavor substances in rice wine were determined based on the combination of olfactometer and temperament. Food Ind. 2011, 32, 102–105. [Google Scholar]
- Li, D.; Yang, M.; Wen, X.; Wu, Y.; Wang, Z.; Geng, J. Research progress on volatile flavor components of rice wine. Food Res. Dev. 2022, 43, 202–207. [Google Scholar]
- Van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter: Houten, The Netherlands, 2003. [Google Scholar]






| Strain | EC Content (g/L) | Degradation Rate (%) |
|---|---|---|
| Control | 2.50 ± 0.03 | - |
| Cl-2 | 2.29 ± 0.05 | 8.4% |
| Wc | 2.11 ± 0.03 | 15.6% |
| Cl-p | 1.31 ± 0.04 | 47.69% |
| Yq | 1.98 ± 0.02 | 20.8% |
| Fermenting Property | Bran Qu |
|---|---|
| liquefaction power | 0.45 ± 0.01 mg/(g·h) |
| Saccharification power | 271 ± 25.51 mg/(g·h) |
| esterification power | 30.78 ± 0.29 mg/(g·100 h) |
| Compounds | Control (mg/L) | Cl-p (mg/L) |
|---|---|---|
| 2-methyl-1-Propanol | - | 0.63 ± 0.02 |
| 3-methyl-1-Butanol | - | 1.95 ± 0.05 |
| 2,3-Butanediol | 1.78 ± 0.03 | 2.21 ± 0.03 |
| 2-Furanmethanol | 1.97 ± 0.05 | 1.77 ± 0.03 |
| 2-Phenylethyl Alcohol | - | 6.88 ± 0.03 |
| 1-Propanol | 1.41 ± 0.06 | - |
| Glycerin | 10.44 ± 0.10 | 10.25 ± 0.04 |
| dl-Glyceraldehyde dimer | 13.70 ± 0.25 | - |
| Ethyl Acetate | - | 4.80 ± 0.02 |
| 2-Hydroxyacetic acid ethyl est | - | 0.11 ± 0.01 |
| Methyl 6-oxoheptanoate | - | 0.45 ± 0.02 |
| Isosorbide Dinitrate | 0.56 ± 0.04 | 0.50 ± 0.01 |
| Cycloserine | 0.26 ± 0.04 | - |
| Acetic acid | 5.60 ± 0.14 | 4.20 ± 0.01 |
| Formic acid | 1.91 ± 0.05 | 1.05 ± 0.02 |
| O-(phenylmethyl)-L-Serine | 0.37 ± 0.02 | - |
| O-Acetyl-L-serine | 0.77 ± 0.04 | - |
| 3-hydroxy-Dodecanoic acid | 0.24 ± 0.02 | 0.29 ± 0.01 |
| Muramic acid | 5.54 ± 0.19 | - |
| Alanine | - | 0.06 ± 0.001 |
| î-N-Formyl-L-lysine | - | 0.22 ± 0.01 |
| 5-(hydroxymethyl)-2-Furancarboxaldehyde | 2.97 ± 0.08 | 2.06 ± 0.02 |
| 3-(methylthio)-Propanal | - | 0.16 ± 0.02 |
| Phenylacetaldehyde | - | 0.53 ± 0.02 |
| 5-methyl-2-Furancarboxaldehyde | 0.50 ± 0.02 | - |
| Furfural | 0.95 ± 0.03 | 0.33 ± 0.02 |
| hydroxy-Acetaldehyde | 1.16 ± 0.01 | 0.68 ± 0.02 |
| 2-methyl-Propanal | 0.89 ± 0.02 | 1.33 ± 0.03 |
| Acetaldehyde | - | 1.14 ± 0.03 |
| 3-hydroxy-2-Butanone | - | 0.96 ± 0.02 |
| 1-hydroxy-2-Propanone | 0.63 ± 0.04 | 0.69 ± 0.01 |
| 2-hydroxy-2-Cyclopenten-1-one | 1.03 ± 0.02 | 0.83 ± 0.02 |
| 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-Pyran-4-one | 1.11 ± 0.02 | 0.89 ± 0.02 |
| 1,3-dihydroxy-2-Propanone | - | 1.31 ± 0.02 |
| (R*,R*)-(ñ)-2,2′-Bioxirane | 1.05 ± 0.02 | 0.84 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liu, J.; Wang, H.; Gou, F.; He, Y.; Yang, L. Advancements in Fermented Beverage Safety: Isolation and Application of Clavispora lusitaniae Cl-p for Ethyl Carbamate Degradation and Enhanced Flavor Profile. Microorganisms 2024, 12, 882. https://doi.org/10.3390/microorganisms12050882
Zhao Y, Liu J, Wang H, Gou F, He Y, Yang L. Advancements in Fermented Beverage Safety: Isolation and Application of Clavispora lusitaniae Cl-p for Ethyl Carbamate Degradation and Enhanced Flavor Profile. Microorganisms. 2024; 12(5):882. https://doi.org/10.3390/microorganisms12050882
Chicago/Turabian StyleZhao, Yingchun, Jun Liu, Han Wang, Fayuan Gou, Yiwei He, and Lijuan Yang. 2024. "Advancements in Fermented Beverage Safety: Isolation and Application of Clavispora lusitaniae Cl-p for Ethyl Carbamate Degradation and Enhanced Flavor Profile" Microorganisms 12, no. 5: 882. https://doi.org/10.3390/microorganisms12050882